Chemical bonding II
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1240
In this section we will analyze the geometry of the molecules, using the valence shell electron pair repulsion theory VSEPR. This will allow us to know the spatial distribution of the atoms in the molecule, a fundamental issue to predict physical properties and reactivity of chemical compounds.
Secondly, we will develop the valence bond theory (VTE), which will help us understand how covalent bonds are formed between the atoms of a molecule. This theory uses the concept of hybridization to explain the experimental facts.
Finally, we will describe in great detail the theory of molecular orbitals (TOM), based on quantum mechanics, allows us to explain certain experimental data, impossible to explain with the TEV.
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1180
In this section we will study how the atoms that make up a molecule are arranged in space. This spatial arrangement (molecular geometry) affects the physicochemical properties of the substance (melting and boiling point, solubility.....)
Although the determination of molecular geometry must be done experimentally, there is a simple method for losing molecular geometry, called the valence shell electron pair repulsion (VSEPR) method. This method is based on arranging the bonds, which start from a central atom, so that the repulsions between the bonding electrons are as low as possible.
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1741
They are AB 2 type molecules in which the central atom has no lone pairs. To avoid repulsions between the bonding pairs, arrange the B groups as far apart as possible. 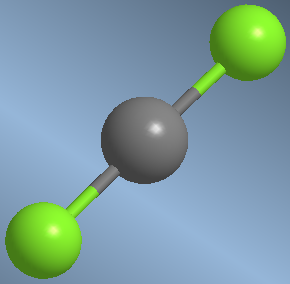 An example is beryllium dichloride, BeCl 2 .
An example is beryllium dichloride, BeCl 2 . ![]()
The molecule is linear with bond angles of 180º.
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1364
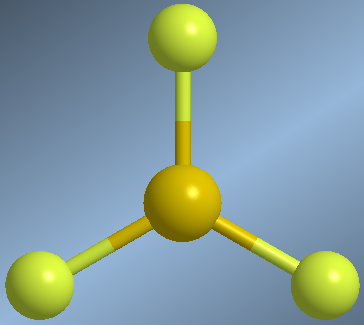 Molecules that form three covalent bonds, without lone pairs on the central atom, arrange the AB bonds towards the vertices of an equilateral triangle, calling this flat trigonal geometry
Molecules that form three covalent bonds, without lone pairs on the central atom, arrange the AB bonds towards the vertices of an equilateral triangle, calling this flat trigonal geometry
As an example we will analyze the geometry of boron trifluoride, BF 3 . 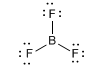
The BF bonds are arranged towards the vertices of an equilateral triangle, with bond angles of 120º.
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1691
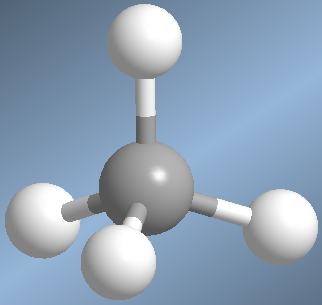 Molecules with four bonds arrange the groups toward the vertices of a tetrahedron. In this geometry the bond angles are 109.5º.
Molecules with four bonds arrange the groups toward the vertices of a tetrahedron. In this geometry the bond angles are 109.5º.
An example of this spatial arrangement is found in methane, CH 4 
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1379
 When a central atom joins 5 groups, the spatial arrangement that minimizes the repulsions between the bonding pairs is the trigonal dipyramid.
When a central atom joins 5 groups, the spatial arrangement that minimizes the repulsions between the bonding pairs is the trigonal dipyramid.
Let's look at the example of phosphorous pentachloride, PCl 5 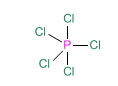
The atoms located in the triangular plane are called equatorial and those above and below this plane, axial. Equatorial chlorines have bond angles of 120º. Between an axial and an equatorial chlorine the angle is 90º.
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1818
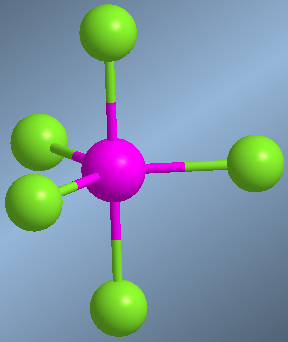
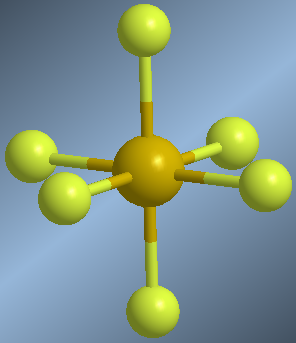 Molecules whose central atom is surrounded by 6 bonding pairs are arranged with octahedral geometry. This spatial arrangement allows minimizing the repulsions between the 6 bonding pairs and is equivalent to a square dipyramid. Atoms located in an equatorial position form 90º angles with each other. Atoms in axial position also form angles of 90º with respect to the equatorial ones and 180º between them.
Molecules whose central atom is surrounded by 6 bonding pairs are arranged with octahedral geometry. This spatial arrangement allows minimizing the repulsions between the 6 bonding pairs and is equivalent to a square dipyramid. Atoms located in an equatorial position form 90º angles with each other. Atoms in axial position also form angles of 90º with respect to the equatorial ones and 180º between them.
An example of this geometry occurs in sulfur hexafluoride, SF 6 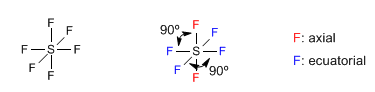
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1271
For molecules whose central atom has lone pairs, we will use the ABE notation, where A is the central atom, B is a surrounding atom, and E is a lone pair of A.
Lone Pair Repulsion> Lone-Bonding Pair Repulsion> Bonding Pair Repulsion
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1630
 Molecules that have a lone pair on the central atom and two bonding pairs have an angular geometry.
Molecules that have a lone pair on the central atom and two bonding pairs have an angular geometry.
An example of this geometry is found in sulfur dioxide, SO 2 
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1980
They are molecules with two binding pairs and two lone pairs. These four groups are arranged towards the vertices of a  tetrahedron. However, to give the geometry only bonding pairs (not lone pairs) are taken into account and therefore the geometry of these molecules is angular.
tetrahedron. However, to give the geometry only bonding pairs (not lone pairs) are taken into account and therefore the geometry of these molecules is angular.
An example of this type is found in water, H 2 O 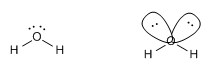
The HOH angle is 104.5º, due to the repulsion of the lone pairs on the bonding pairs.
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1377
They are molecules that have three binding pairs and a lone pair surrounding a central atom. The spatial arrangement 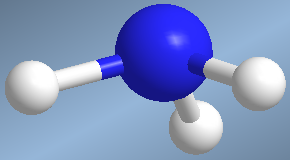 of these four pairs is tetrahedral, however, by neglecting the lone pair, we are left with a pyramidal geometry.
of these four pairs is tetrahedral, however, by neglecting the lone pair, we are left with a pyramidal geometry.
One such molecule is ammonia, NH 3 
The lone pair strongly repels the bonders and the HNH bond angle is less than 109°.
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1955
 Molecules with four binding pairs and one lone pair are arranged in the form of a trigonal dipyramid. However, removing the lone pair results in a kind of distorted tetrahedron.
Molecules with four binding pairs and one lone pair are arranged in the form of a trigonal dipyramid. However, removing the lone pair results in a kind of distorted tetrahedron.
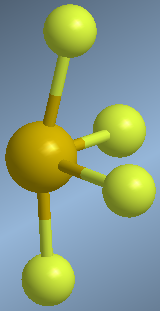
One molecule that exhibits this spatial distribution of groups is sulfur tetrafluoride, SF 4 . 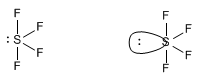
Observe how in the molecular model the axial sulfurs are bent to the right, due to the repulsions with the lone pair.
In the proposed geometry, the para solitaire occupies the equatorial position, undergoing repulsions with two bonding pairs (axial) that are at 90º. If we had placed the lone pair in an axial position, the interactions would have been with three bonding pairs at 90º. 
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 2170
Molecules with three binding pairs and two lone pairs are arranged in a T-shape. The geometry that minimizes the 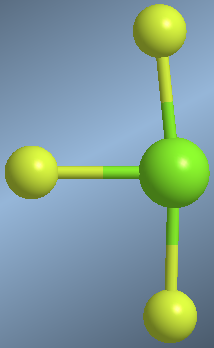 repulsions is the one that arranges the lone pairs in equatorial. Considering the lone pairs, the geometry is a trigonal dipyramid and removing the lone pairs gives us the T shape.
repulsions is the one that arranges the lone pairs in equatorial. Considering the lone pairs, the geometry is a trigonal dipyramid and removing the lone pairs gives us the T shape.
A molecule that responds to this geometry is chlorine trifluoride, ClF 3

As can be seen in the model, the axial fluorines bend slightly to the right due to the repulsions with the lone pairs.
By removing the lone pairs from the drawing, the trigonal dipyramid becomes a T.
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 2132
Molecules with two binding pairs and three lone pairs are arranged in a trigonal bipyramidal shape with the three pairs ![]() lonely in equatorial Without taking into account the lone pairs we obtain a linear geometry.
lonely in equatorial Without taking into account the lone pairs we obtain a linear geometry.
An example of this geometry is found in the triiodide ion, I 3 - 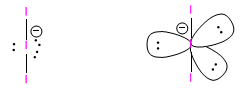
The location of the lone pairs in equatorial minimizes the repulsions between them, as they are located at 120º.
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 2379
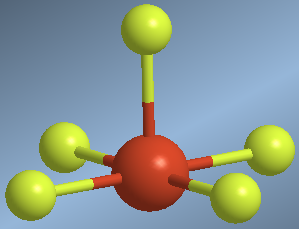 They are molecules with five binding pairs and one solitary pair. The spatial layout including the lone pair is octahedral, without considering the lone pair we are left with a square pyramid.
They are molecules with five binding pairs and one solitary pair. The spatial layout including the lone pair is octahedral, without considering the lone pair we are left with a square pyramid.
An example of this geometry is found in the bromine pentafluoride molecule, BrF 5 
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 3386
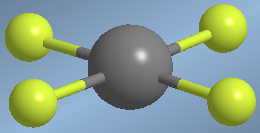 Molecules with four bonding pairs and two lone pairs present an octahedral spatial arrangement that becomes a square plane when lone pairs are not considered.
Molecules with four bonding pairs and two lone pairs present an octahedral spatial arrangement that becomes a square plane when lone pairs are not considered.
An example of this spatial arrangement is found in xenon tetrafluoride, XeF 4 
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1337
The VSEPR model allows us to determine with great accuracy the geometry of a molecule. Lewis's theory allows us to understand how a covalent bond is formed through the pairing of electrons. However, we cannot with these theories explain the lengths and energies of the bonds formed. Thus, an HH bond is very strong and short (436.4 kJ/mol and 74 pm), compared to the much weaker and long FF bond (150.6 kJ/mol and 142 pm).
To explain these properties it is necessary to use theories based on quantum mechanics, such as the valence bond theory, which considers electrons in atomic orbitals, which when overlapping form bonds. The characteristics of these bonds depend on the type of orbitals that overlap (size and geometry), which explains the great difference between the properties of HH and FF bonds.
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1392
According to Lewis's theory, the hydrogen molecule is formed by pairing the pair of electrons contributed by each hydrogen atom.
In the valence bond theory, the bond is produced by overlapping of the 1s orbitals of each hydrogen atom. This overlap means that both orbitals share the same region of space. 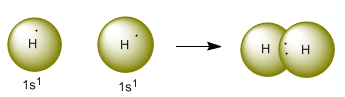
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1299
The Lewis theory and the VSEPR model have given us a fairly accurate description of the structure and geometry of the molecule. ![]()
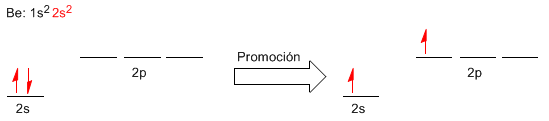
Now we will try to better understand the formation of Be-Cl bonds using the valence bond theory. For this we will write the electronic configuration of beryllium, looking at the valence layer.