First Principle | Thermodynamics
- Details
- Written by: Germán Fernández
- Category: First Law of thermodynamics
- Hits: 1187
In this section we will study a new state function, the internal energy U, and its relationship to heat and work. Another important state function in chemistry is the enthalpy H. will also be introduced the heat capacities at constant pressure and volume, Cp and Cv, which give us the variations of internal energy and enthalpy with temperature.
- Details
- Written by: Germán Fernández
- Category: First Law of thermodynamics
- Hits: 1797
As in classical mechanics, work is defined by the equation dw = F x dx, where F x is the component of force F in the direction of motion and dx is the displacement. When F x and dx have the same sense, the work is positive, if they have opposite directions the work is negative.
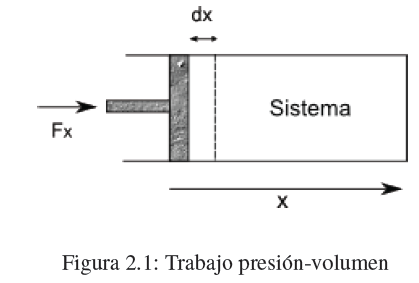
Consider a cylinder with a piston acted on by a force Fx producing a piston displacement dx. The work done on the system is given by the equation, dw =F x dx. Let A be the area of the piston, the pressure exerted by the piston on the system will be, P=F x /A, substituting in the work expression, dw = PAdx.
The product Adx represents the volume variation produced by the movement of the piston dV=-Adx. Substituting in dw we get: ![]()
If we do work on the system (compression) dV<0 and the work is positive. When the system does work on the surroundings (expansion) dV>0 work is negative.
- Details
- Written by: Germán Fernández
- Category: First Law of thermodynamics
- Hits: 1187
Heat is the second form of energy transfer that we are going to study. When two bodies at different temperatures come into contact through a thermally conductive wall, a flow of heat is produced from the hot body to the cold one until the temperatures are equal.
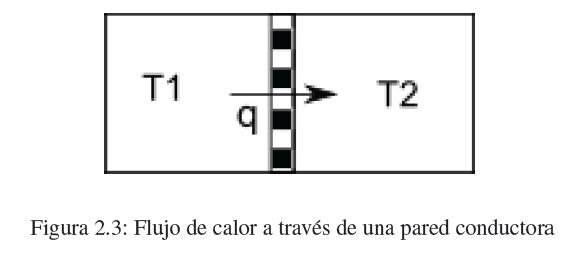
The amount of heat required to raise the temperature of the system by one dT is given by the expression:
![]()
where m is the mass of the system and c p its specific heat at constant pressure.
- Details
- Written by: Germán Fernández
- Category: First Law of thermodynamics
- Hits: 1400
How can we change the internal energy of a system? The change of internal energy of a system is produced by exchanges of heat and/or work. When we do work or add heat to a system, we increase its internal energy. If the system loses heat or does work on the surroundings, its internal energy decreases.
- Details
- Written by: Germán Fernández
- Category: First Law of thermodynamics
- Hits: 1241
The enthalpy H of a thermodynamic system is defined as: ![]()
The importance of this magnitude in chemistry lies in the fact that its variation coincides with the heat exchanged at constant pressure. It is very common for a chemical reaction to take place in an open vessel (at atmospheric pressure) and the heat exchanged will be an enthalpy change.
We start from the first law of thermodynamics: ![]() , the subscript p indicates constant pressure.
, the subscript p indicates constant pressure. 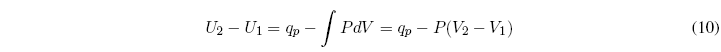
Clearing the heat and grouping terms we obtain: 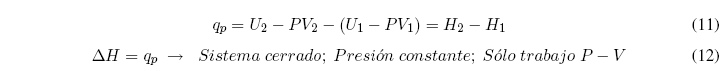
- Details
- Written by: Germán Fernández
- Category: First Law of thermodynamics
- Hits: 1211
The heat capacity of a closed system in an infinitesimal process is defined as the ratio between the heat exchanged and the temperature change produced.
For a process at P=cte, the heat capacity is given by the equation: 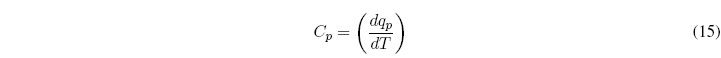
The heat capacity at constant volume is defined in the same way: 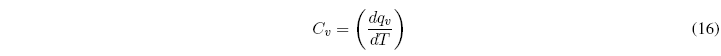
As ![]() , the above equations can be written as:
, the above equations can be written as: 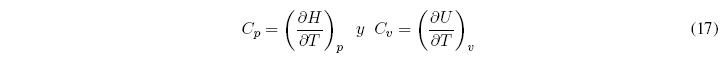
Therefore, C p and C v give us the variation of U and H with respect to temperature and must always take positive values.
We define molar heat capacities for pure substances as the ratio between C p and the number of moles of the substance. 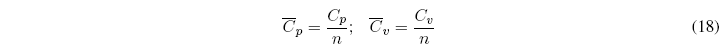
- Details
- Written by: Germán Fernández
- Category: First Law of thermodynamics
- Hits: 1269
An ideal gas satisfies the equation of state PV = nRT. The interactions between gas molecules are negligible, that is, there are no intermolecular forces. In an ideal gas, the change in volume does not affect the internal energy as long as the temperature is kept constant. A decrease in volume causes the molecules to be closer, but since there are no interactions between them, the internal energy does not change.
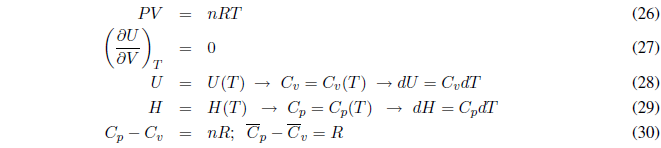
- Details
- Written by: Germán Fernández
- Category: First Law of thermodynamics
- Hits: 1272
During this process the temperature is kept constant. Since the internal energy of ideal gases only depends on temperature: \(\Delta U=0\) and \(\Delta H=0\).
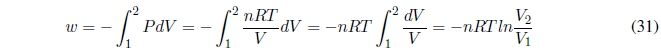
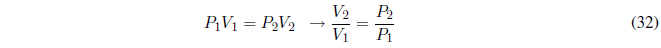
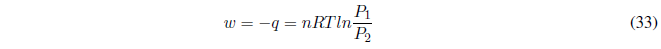
- Details
- Written by: Germán Fernández
- Category: First Law of thermodynamics
- Hits: 1396
We start calculating the work, since P is constant it goes out of the integral.
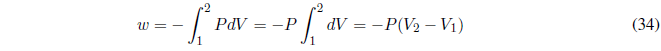
AP constant the heat exchanged is given by the enthalpy change.
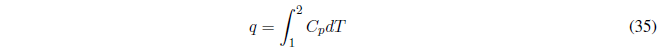
- If \(C_p\) is independent of temperature it can be taken from the integral.
- If we are given as data \(C_v\) instead of \(C_p\) remember the relationship between both magnitudes, \(C_p-C_v=R\).
- The first principle gives us the internal energy change in the process, \(\Delta U =q+w\) .
- The enthalpy change coincides with the heat exchanged.\(\Delta H =q\)
- Details
- Written by: Germán Fernández
- Category: First Law of thermodynamics
- Hits: 1157
AV constant the work done is null \(w=0\).
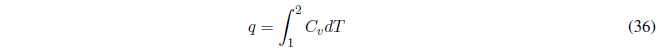
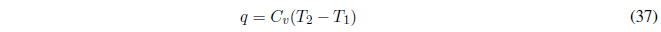
- Details
- Written by: Germán Fernández
- Category: First Law of thermodynamics
- Hits: 1125
In an adiabatic process, the walls of the system prevent heat exchange, therefore, \(dq=0\;\;\rightarrow dU=dw\).
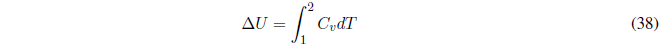
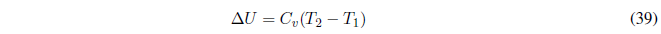
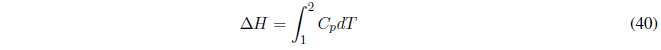
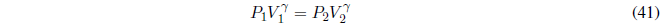
- Details
- Written by: Germán Fernández
- Category: First Law of thermodynamics
- Hits: 1508
When a thermodynamic system goes from state 1 to state 2, it is said to have undergone a thermodynamic process. The intermediate thermodynamic states that lead from state 1 to 2 are called trajectories or paths. Two processes with different trajectories can connect the same initial and final states.
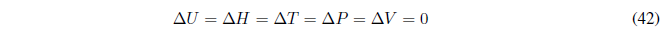
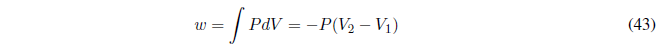
Read more: Calculation of q, w, U and H in thermodynamic processes



