In an adiabatic process, the walls of the system prevent heat exchange, therefore, \(dq=0\;\;\rightarrow dU=dw\).
The internal energy variation can be calculated with:
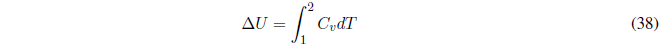
if \(C_v\) remains constant,
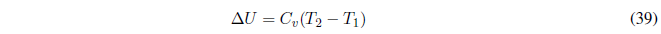
The enthalpy change is calculated with:
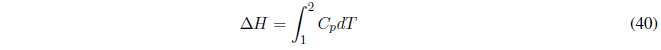
Remember that in adiabatic processes the equations must be used:
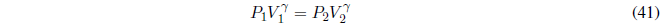
where \(\gamma=\frac{C_p}{C_v}\)



