The atom, molecules and ions
- Details
- Written by: Germán Fernández
- Category: The atom, molecules and ions
- Hits: 1319
For 2000 years there has been speculation about the hypothesis that gives the atom the basic unit that constitutes all matter. For about 200 years, chemists have established different atomic models in which the atom is considered to be made up of protons, neutrons, and electrons. The models that have had the greatest impact consider that protons and neutrons form a high-mass nucleus around which electrons revolve.
In the last 30 years, powerful microscopes (tunneling microscope) capable of visualizing individual atoms have been developed, irrefutable proof of the validity of the atomic theory.
Man used and dominated chemical reactions in prehistory, an example is combustion (fire). Obtaining iron from its ores dates from the year 1300 BC. C. and many important chemicals, such as nitric acid (strong water), sulfuric acid (vitriol oil) and ammonium sulfate (Glauber's salt) have been used for hundreds of years.
However, the fundamental principles that govern these reactions and allow us to explain the physical and chemical properties of substances were not established until very recent times.
- Details
- Written by: Germán Fernández
- Category: The atom, molecules and ions
- Hits: 1666
The Greek philosopher Democritus, in the 5th century BC, hypothesized that matter was made up of small, indivisible particles which he called atoms.
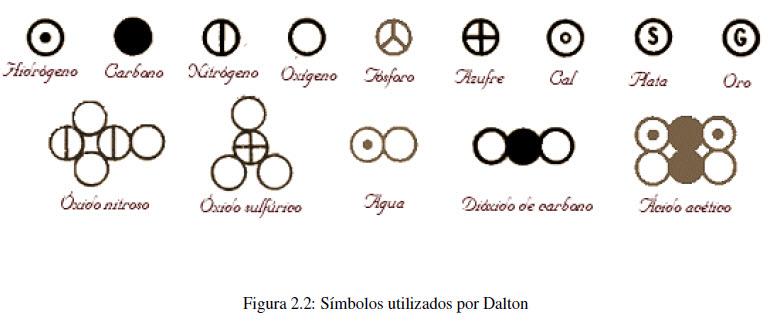
In 1808, the English scientist, John Dalton, took up the ideas of Democritus, formulating them in a more precise way. The hypotheses on which Dalton's theory is based can be summarized in three points:
- Elements are made up of very small particles called atoms. The atoms of an element are identical (same mass, chemical properties) but different from the atoms of other elements.
- Compounds are formed by the union of atoms of two or more elements. The ratio between the number of atoms present in a compound is always a whole number or a simple fraction.
- In chemical reactions, atoms are separated, combined, or regrouped, never created or destroyed.
- Details
- Written by: Germán Fernández
- Category: The atom, molecules and ions
- Hits: 1561
 Dalton in his atomic theory considers the atom as the basic unit of an element that can take part in a chemical reaction. For Dalton the atom was indivisible. However, work carried out after 1850 showed that atoms are made up of simpler particles, called subatomic particles.
Dalton in his atomic theory considers the atom as the basic unit of an element that can take part in a chemical reaction. For Dalton the atom was indivisible. However, work carried out after 1850 showed that atoms are made up of simpler particles, called subatomic particles.
The first evidence for atomic structure was provided in the early 1800s by the English chemist Humphry Davy (1778-1829). Davy found that electric current decomposed certain substances, suggesting that the elements of a compound were held together by electrical forces. In 1832 Michael Faraday (1791-1867) determined the amount of current needed to perform the electrolysis of a substance. In later studies together with George Stoney (1826-1911) they led Faraday to relate the unit of electric charge, which he called the electron, with the atom.
- Details
- Written by: Germán Fernández
- Category: The atom, molecules and ions
- Hits: 1680
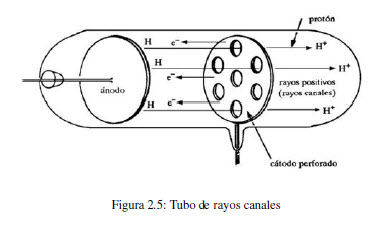
In 1886, Eugen Goldstein (1850-1930) observed that in a cathode ray tube, with a perforated anode, a stream of particles was generated moving from the cathode to the anode. These positive rays come from atoms contained in the tube that have lost electrons. By changing the gas contained in the tube, a change in the e/m ratio of the positive particle is observed. Studies carried out with different gases showed that the charge of ions is a multiple of a value, the unit of positive charge, called a proton.
- Details
- Written by: Germán Fernández
- Category: The atom, molecules and ions
- Hits: 1351
 In 1895, the German physicist Wilhelm Röntgen observed that by shining cathode rays on metal plates, they emitted high-energy rays, which due to their unknown nature he called X-rays.
In 1895, the German physicist Wilhelm Röntgen observed that by shining cathode rays on metal plates, they emitted high-energy rays, which due to their unknown nature he called X-rays.
X-rays were not deflected by electric or magnetic fields, which implies that they are not made up of charged particles. They were also capable of passing through matter, obscuring covered photographic plates.
In 1896, Antoine Becquerel, professor of physics in Paris, observed how certain uranium minerals naturally gave off radiation similar to X-rays. Marie Curie proposed the name radioactivity to describe the phenomenon, calling radioactive materials substances that present this property.
- Details
- Written by: Germán Fernández
- Category: The atom, molecules and ions
- Hits: 1709
 By the early 1900s chemists knew that the atom contained negatively charged particles (electrons) and positive particles (protons), the only remaining question was how to distribute them in the atom.
By the early 1900s chemists knew that the atom contained negatively charged particles (electrons) and positive particles (protons), the only remaining question was how to distribute them in the atom.
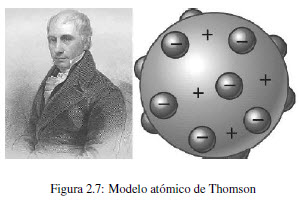
JJ Thomson proposed an atomic model that consisted of uniformly distributing negative particles within a positively charged sphere. This model was called "raisin pudding" because of its resemblance to the famous English dessert.
- Details
- Written by: Germán Fernández
- Category: The atom, molecules and ions
- Hits: 1329
In 1910, the New Zealand physicist Ernest Rutherford (1871-1937) together with his colleague Hans Geiger and a student named Ernest Marsden bombarded a thin sheet of gold with alpha particles from a radioactive source. They used a zinc sulfide fluorescent screen to determine the trajectory of the particles after the collision. They observed that most of the particles passed through the sheet without deviating, some suffered a slight variation in their trajectory, but a small fraction (0.001%) were deflected by a significant angle, even observing some rebounds.
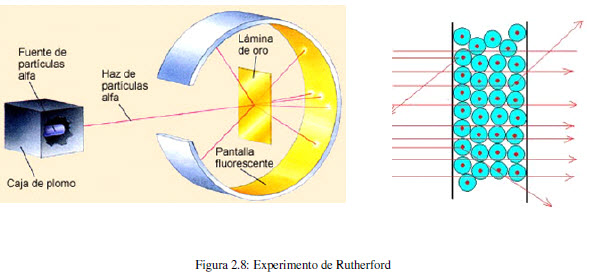
This experiment is incompatible with Thomson's model, since the uniform distribution of the charge prevents explaining such important changes in the trajectory of the alpha particles.
- Details
- Written by: Germán Fernández
- Category: The atom, molecules and ions
- Hits: 1419
The third fundamental particle is the neutron, discovered in 1932 by James Chadwick (1891-1974) by bombarding a sheet of beryllium with alpha particles, he observed the emission by the metal of very high energy radiation, similar to gamma rays. Later studies showed that said radiation was made up of neutral particles (they do not respond to electric fields) with a mass slightly greater than that of protons.
The discovery of the neutron allowed us to understand the reason why the helium atom has a mass 4 times greater than that of hydrogen, containing only two protons. The explanation lies in the existence of 2 neutrons in its nucleus.
- Details
- Written by: Germán Fernández
- Category: The atom, molecules and ions
- Hits: 1364
The atomic number (Z) is the number of protons possessed by the nucleus of the atom of a chemical element. If the atom is neutral, it also coincides with the number of electrons it has in the shell. For example, oxygen has atomic number 8 (Z=8), this tells us that it has 8 protons in its nucleus and 8 electrons in its crust.

In 1913, HGJ Moseley (1887-1915) found that the wavelength of X-rays emitted by an element was directly related to the element's atomic number.
The number of protons in the nucleus of an atom determines its identity, this number is known as the atomic number of the element.
- Details
- Written by: Germán Fernández
- Category: The atom, molecules and ions
- Hits: 1182
The mass number (A) is the number of protons and neutrons present in the nucleus of the atom of a chemical element. There is a notation that allows to represent the atomic and mass number of an element: $^A_ZX$ Knowing the atomic and mass numbers of an element, the number of neutrons that the nucleus of one of its atoms will have can be deduced: neutrons = A - Z
Atoms of an element (same atomic number) that have different mass numbers are called isotopes . Thus, hydrogen has three isotopes. Hydrogen has a proton in the nucleus, no neutrons (atomic number 1, mass number 1). Deuterium has one proton and one neutron (atomic number 1, mass number 2). The third isotope of hydrogen is called tritium, it has one proton and two neutrons (atomic number 1, mass number 3). All isotopes of an element have the same atomic number and differ in mass number.
- Details
- Written by: Germán Fernández
- Category: The atom, molecules and ions
- Hits: 1261
We can think that to determine the mass of an atom we only have to add the masses of the particles that constitute it (protons, neutrons and electrons). However, when the nucleus is formed, a significant amount of energy is released, called nuclear energy, which implies a decrease in the mass of the nucleus with respect to the sum of the masses of the particles that compose it.
Therefore, the determination of atomic masses must be done experimentally. First, the carbon-12 atom is chosen as the standard and assigned a mass of 12 atomic mass units (amu). Next, the mass of the remaining atoms is determined with respect to carbon-12 using a mass spectrophotometer.
For example, data from a mass spectrum indicate that the ratio of the masses of \(^{16}O\) to \(^{12}C\) is 1.33291. That is, oxygen has a mass 1.33291 times greater than carbon, the mass of oxygen will be given by: \(1.33291\;x\;12=15.9949\;uma\)
- Details
- Written by: Germán Fernández
- Category: The atom, molecules and ions
- Hits: 1241
With what was discussed in the previous point, we are surprised to see that the mass of carbon is 12,011 amu, since a standard mass of 12 amu was assigned to it. The reason for this apparent inconsistency is the presence of isotopes: carbon-12, carbon-13 and carbon-14. The atomic mass of carbon is obtained by means of the weighted average of the masses of each isotope, that is, multiplying the abundance of each isotope by its mass and adding this result for all the isotopes.
- Details
- Written by: Germán Fernández
- Category: The atom, molecules and ions
- Hits: 1467
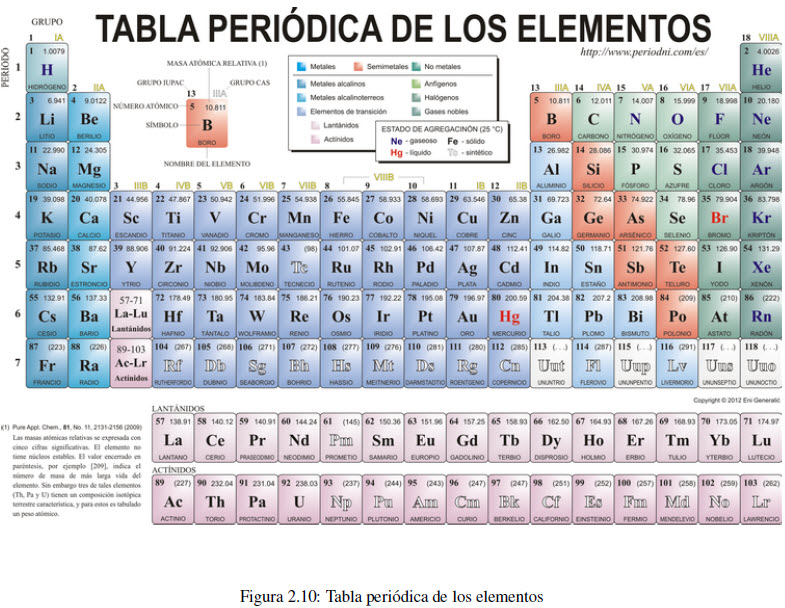
The periodic table arranges the elements by increasing atomic number, starting at the top left. Elements located in the same column (group) have similar properties. For example, group 1 includes the alkali metals (lithium, sodium, potassium, rubidium and cesium), all of which are good conductors of heat and electricity, and have a violent reaction with water. Group 2 is made up of the alkaline earth metals (beryllium, magnesium, calcium, strontium, barium, and radium). The elements of group 17 are called halogens (fluorine, chlorine, bromine and iodine) and those of group 18 noble gases (helium, neon, argon, krypton, xenon and radon).