In 1910, the New Zealand physicist Ernest Rutherford (1871-1937) together with his colleague Hans Geiger and a student named Ernest Marsden bombarded a thin sheet of gold with alpha particles from a radioactive source. They used a zinc sulfide fluorescent screen to determine the trajectory of the particles after the collision. They observed that most of the particles passed through the sheet without deviating, some suffered a slight variation in their trajectory, but a small fraction (0.001%) were deflected by a significant angle, even observing some rebounds.
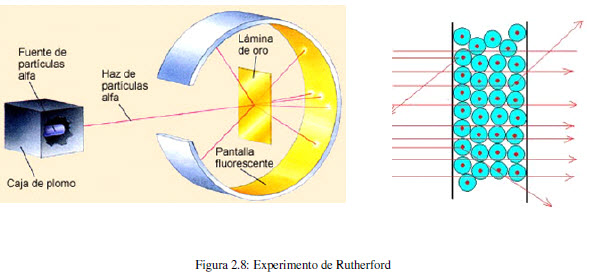
This experiment is incompatible with Thomson's model, since the uniform distribution of the charge prevents explaining such important changes in the trajectory of the alpha particles.
This experiment left Rutherford and coworkers stunned. Rutherfor commented: "It's as if you had fired a 15-inch bullet at a piece of tissue paper and the bullet ricocheted off."
The analysis of the results of the experiment led Rutherford to create an atomic model in which the positive particles (protons) form a small nucleus of high mass, responsible for the deflections observed in the experiment. Electrons revolve around the nucleus at great distances.



