Thermochemistry
- Details
- Written by: Germán Fernández
- Category: thermochemistry
- Hits: 1292
Thermochemistry is the field of thermodynamics dedicated to the study of energy exchanges that take place during a chemical reaction. It also allows predicting the spontaneity of a reaction.
On the right margin, a thermite reaction between aluminum and iron (III) oxide can be observed with a significant release of heat (strongly exothermic reaction).
- Details
- Written by: Germán Fernández
- Category: thermochemistry
- Hits: 1534
 Thermochemistry is the part of thermodynamics that deals with energy exchanges in chemical reactions. Although there are also numerous physical processes that involve heat transfer, such as phase changes and the formation of solutions.
Thermochemistry is the part of thermodynamics that deals with energy exchanges in chemical reactions. Although there are also numerous physical processes that involve heat transfer, such as phase changes and the formation of solutions.
When a chemical reaction or physical process releases heat, it is said to be exothermic. A process will be endothermic when it absorbs heat. The thermodynamic magnitude that measures these energy exchanges is called enthalpy (heat at constant pressure), represented by $\Delta H$
- Details
- Written by: Germán Fernández
- Category: thermochemistry
- Hits: 1531
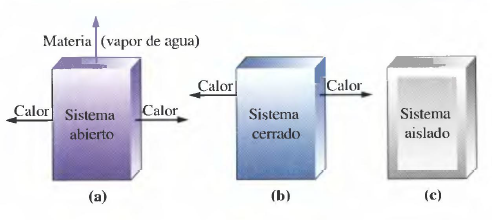 In thermochemistry we will use the following concepts:
In thermochemistry we will use the following concepts:
- System: part of the universe under study.
- Part of the universe that interacts with the system.
- Open system: can exchange matter and energy with the environment.
- Closed system: can exchange energy, but not matter, with the environment.
- Isolated system: does not exchange matter or energy.
- Details
- Written by: Germán Fernández
- Category: thermochemistry
- Hits: 1296
Heat is a form of energy exchange between the system and its surroundings due to a temperature difference. Thermodynamic systems do not have heat, they have energy, and one of the ways in which they exchange this energy is heat.
Heat always flows from the higher-temperature body to the lower-temperature body until thermal equilibrium is reached, at which time the temperatures of both bodies are equal.
Heat transfer can not only cause changes in temperature, it can also lead to changes in the state of aggregation of matter, fusion of solids, vaporization of liquids... During these phase changes, the temperature remains constant, using the heat energy in overcoming the forces of interaction between the molecules that make up the solid, in the case of fusion. In vaporization, heat energy is used to overcome the forces between liquid molecules and allow them to pass into the vapor phase.
- Details
- Written by: Germán Fernández
- Category: thermochemistry
- Hits: 1261
I am going to illustrate how to determine the specific heat of a substance, through a very visual procedure, exposed by Ralph Petrucci in his book "General Chemistry".
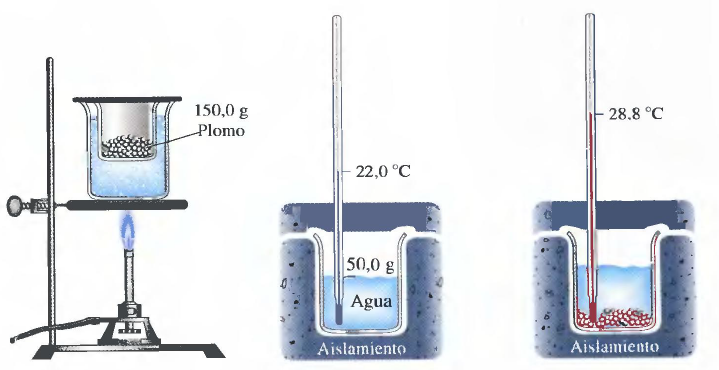
The substance of which we will determine its specific heat is lead. The first step is to heat the lead to a known temperature. A very simple way is to heat it in a water bath, bringing the water to a boil, which guarantees a temperature of 100ºC.



