 In thermochemistry we will use the following concepts:
In thermochemistry we will use the following concepts:
- System: part of the universe under study.
- Part of the universe that interacts with the system.
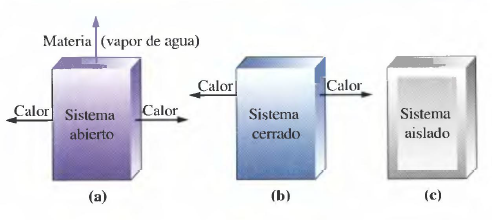
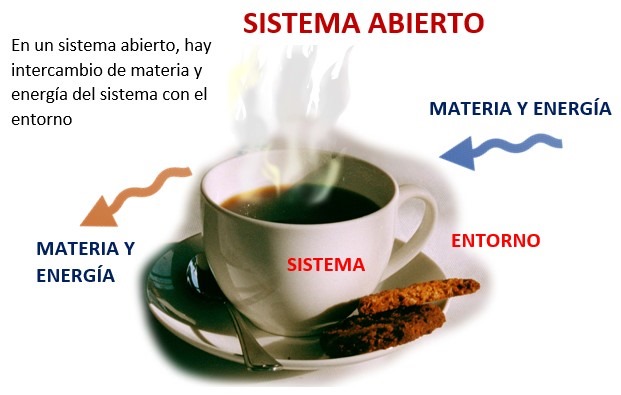
- Open system: can exchange matter and energy with the environment.
- Closed system: can exchange energy, but not matter, with the environment.
- Isolated system: does not exchange matter or energy.
The system walls (separation between system and environment) can be:
- Permeable: allow the passage of matter
- Waterproof: they do not allow the passage of matter.
- Conductive or diathermic: allow the passage of energy.
 Insulating or adiabatic: they do not allow the passage of energy.
Insulating or adiabatic: they do not allow the passage of energy.
Thermodynamic processes can be classified into the following types:
- Isobaric processes: they take place at constant pressure.
- Isothermal processes: they take place at constant temperature
- Isochoric processes: they take place at constant volume.
- Adiabatic processes: they occur without heat transfer between the system and the environment.



