Fundamentals of chemistry
- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1480
 Chemistry is the science that studies matter, its structure, composition, properties, and the physical and chemical processes it undergoes, as well as the energy exchanges that accompany these processes.
Chemistry is the science that studies matter, its structure, composition, properties, and the physical and chemical processes it undergoes, as well as the energy exchanges that accompany these processes.- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1234
 Matter is anything that has mass and occupies space. Mass is the measure of the amount of matter that a body possesses. The force required to accelerate a body increases with its mass (Newton's second law).
Matter is anything that has mass and occupies space. Mass is the measure of the amount of matter that a body possesses. The force required to accelerate a body increases with its mass (Newton's second law).- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1380
 In any physical or chemical process there is no change in the amount of matter. For a chemical reaction, the sum of the masses of the reactants must equal the sum of the masses of the products.
In any physical or chemical process there is no change in the amount of matter. For a chemical reaction, the sum of the masses of the reactants must equal the sum of the masses of the products.- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1239
- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1230
- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1166
 Matter can be classified into three clearly differentiated states.
Matter can be classified into three clearly differentiated states.- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1197
- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1213
- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1331
 Mixtures are combinations of pure substances. Each substance retains its physical and chemical properties in the mixture. For example, if we put 50 ml of methanol and 50 ml of water in a glass, a mixture is obtained, in which both methanol and water retain their properties.
Mixtures are combinations of pure substances. Each substance retains its physical and chemical properties in the mixture. For example, if we put 50 ml of methanol and 50 ml of water in a glass, a mixture is obtained, in which both methanol and water retain their properties.- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1261
 Chemistry is based on observation and experimentation, trying to obtain a series of results that in many cases can be expressed numerically, allowing their comparison with those obtained in other experiences.
Chemistry is based on observation and experimentation, trying to obtain a series of results that in many cases can be expressed numerically, allowing their comparison with those obtained in other experiences.- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1155
In scientific notation the number 0.000005 is expressed as 5 10 -6,
- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1236
The results obtained in a measurement are not exact. Every measurement implies an estimate. For example, suppose we need to measure an object with a ruler graduated in millimeters. When measuring we obtain a result between 38 and 39 millimeters, we estimate that the object measures 38.5 millimeters. This result has an exact part 38 and a part that is estimated (approximate) which is the last digit 5. The number 38.5 mm contains three significant figures. The last digit is doubtful, but is considered a significant figure. When giving the result of a measurement we include an approximate digit, but only one.
Next we will use a measuring cylinder to measure volumes of liquids. To the right of the specimen the calibration lines are enlarged. On the right scale we move from 10 ml to 10 ml. The left scale is graduated so that it varies from milliliter to milliliter. 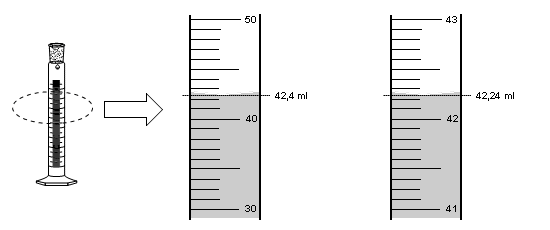
- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1280
- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1227
Suppose that 8.56 is the result of an operation carried out with the calculator. If the number of significant figures is only two, we should return 8.5. But since the third digit is greater than 5, the second digit is rounded to 6. The final result is 8.6.
When the number to be removed is less than 5, the preceding digit does not change. If 5 is the number to be eliminated, the preceding digit is replaced by the nearest even number.
Let's see examples:
8.48 rounds to 8.5; 2.43 rounds to 2.4; 2.45 rounds to 2.4; 2.35 rounds to 2.4.
- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1300
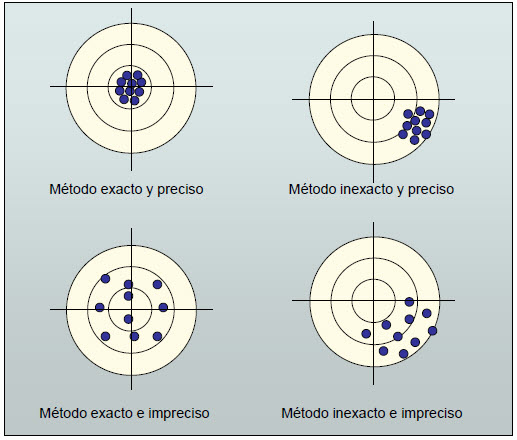
The accuracy gives us the degree of agreement between the measured value and the true one.
The precision is related to the reproducibility of the measurements. Indicates the degree of agreement of several individual measurements.
A scale can be very precise, if when making several measurements it always gives the same result. But it is inaccurate, if that result does not agree with reality. Therefore, measuring instruments must be required to be exact and precise at the same time.
- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1177
The conversion factors are based on multiplying by fractions that have the same quantity in the numerator and denominator but in different units. Some examples of unit factors are the following: 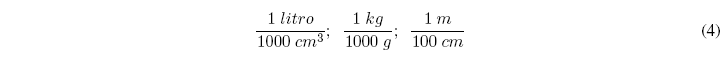
In the conversion factors, the units guide us in the calculations. All the units are canceled until reaching the desired result.
Let's see an application of the conversion factors: Knowing that one erg is equal to 1 10 -7 joules. Convert 3.74 10 -2 ergs to joules.
The solution can be obtained by applying conversion factors: 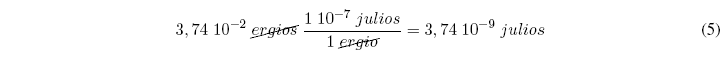
- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1267
Density is defined as mass per unit volume. It is obtained by dividing the mass of a sample by its volume. 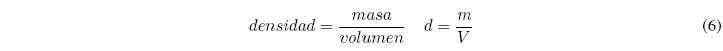
The most used units are g/cm 3 , g/ml and also g/l. Since each substance has a unique density, this information can be used to identify substances.
Relative density relates the density of the substance to that of water, both at the same temperature. 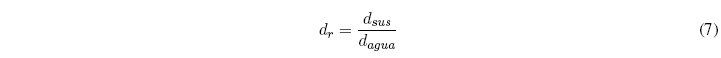
Up to 25ºC we can take 1g/ml as the density of water. Therefore, at room temperature the density of a substance coincides with its relative density. At higher temperatures, the density of water differs from 1g/ml and both magnitudes cease to coincide.
- Details
- Written by: Germán Fernández
- Category: Fundamentals of chemistry
- Hits: 1239
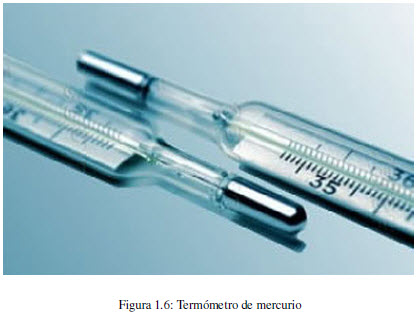 Heat is a form of energy transfer, which occurs by virtue of a temperature difference. The flow of heat always occurs from the hot body to the cold.
Heat is a form of energy transfer, which occurs by virtue of a temperature difference. The flow of heat always occurs from the hot body to the cold.
The temperature of a body can be measured with mercury thermometers, which consist of a reservoir for mercury attached to a capillary. When the tank is heated, the mercury expands through the capillary. The higher the temperature, the higher the mercury rise is observed.
In order to measure temperatures, it is necessary to have a temperature scale. Swedish astronomer Anders Celsius developed the so-called Celsius temperature scale. He takes as points of reference the melting of water, to which he assigns 0º Celsius, and its boiling point at atmospheric pressure, to which he assigns 100º Celsius. Between these points there are one hundred divisions, each representing a degree Celsius.
A widely used temperature scale in the United States is Fahrenheit. On this scale the freezing and boiling points of water are taken to be 32ºF and 212ºF.



