Density is defined as mass per unit volume. It is obtained by dividing the mass of a sample by its volume. 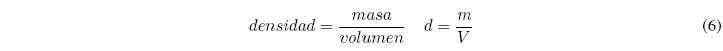
The most used units are g/cm 3 , g/ml and also g/l. Since each substance has a unique density, this information can be used to identify substances.
Relative density relates the density of the substance to that of water, both at the same temperature. 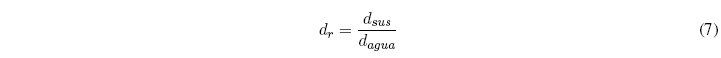
Up to 25ºC we can take 1g/ml as the density of water. Therefore, at room temperature the density of a substance coincides with its relative density. At higher temperatures, the density of water differs from 1g/ml and both magnitudes cease to coincide.



