Periodic properties
- Details
- Written by: Germán Fernández
- Category: periodic properties
- Hits: 1254

- Details
- Written by: Germán Fernández
- Category: periodic properties
- Hits: 1411
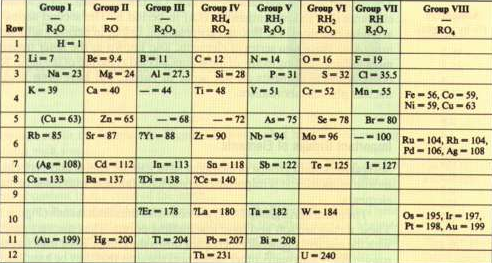
Mendeleev arranged the elements according to atomic masses, leaving blank spaces for elements yet to be discovered. Thus he predicted the existence of the elements of atomic mass 44, 68, 72 and 100 corresponding to scandium, gallium, germanium and technetium.
- Details
- Written by: Germán Fernández
- Category: periodic properties
- Hits: 1468
 Mendeleev arranged the elements according to increasing atomic masses, but he was forced to change the position of some of them to form groups with similar properties. In 1913, Moseley observed that the frequencies of X-rays emitted by an element were related to the number of positive charges present in the nucleus (protons), according to the equation $\nu=A(Zb)^2$, where $\ nu$ the frequency of X-rays, Z the atomic number, A and B constants.
Mendeleev arranged the elements according to increasing atomic masses, but he was forced to change the position of some of them to form groups with similar properties. In 1913, Moseley observed that the frequencies of X-rays emitted by an element were related to the number of positive charges present in the nucleus (protons), according to the equation $\nu=A(Zb)^2$, where $\ nu$ the frequency of X-rays, Z the atomic number, A and B constants.- Details
- Written by: Germán Fernández
- Category: periodic properties
- Hits: 1550

- Details
- Written by: Germán Fernández
- Category: periodic properties
- Hits: 1518
Since the electron density of an atom never goes to zero it is never difficult to define the size of atoms. One possibility is to consider the radius of the sphere that contains a high probability of finding the electron, for example 90$\%$. However, we are more interested in knowing the size of the atoms when they are linked, forming covalent or ionic compounds.
The covalent radius is defined as half the distance between the nuclei of two identical atoms joined by a single covalent bond. The ionic radius is the distance between the nuclei of two atoms joined by an ionic bond. In the latter case, the distance must be distributed correctly between the cation (smaller) and the anion (larger).
- Increase in atomic radius in a group . Increasing the value of n shifts the probability density toward higher values of r, giving rise to a larger atom. A significant increase in radius is observed when passing from Li to Na and from Na to K. The increase in radius in elements with high atomic number (passage from potassium to rubidium) is less due to the existence of d and f subshells that shield the nucleus with less effective than the syp, causing the outer electrons to be more attracted to the nucleus.
- Decrease in atomic radius in a period . Moving to the right in one period produces an increase in the effective nuclear charge that acts on the outer electrons, compressing the atom. In the particular case of the transition elements, this variation is small because the electrons gradually enter an internal (n-1)d subshell that contributes to the shielding of the external electrons, ns.
Read more: Atomic and ionic radius. Variation in the periodic table
- Details
- Written by: Germán Fernández
- Category: periodic properties
- Hits: 1524
Ionization energy, I, is the energy required to remove an electron from a gaseous atom, isolated and in its ground state. The electrons are attracted to the nucleus and it is necessary to provide energy to start them up. The electrons in the last shell are always lost, as they are the ones that are weakest attracted to the nucleus. \begin{equation} Mg(g) \rightarrow Mg^{+}(g)+1e^{-} \end{equation} This equation represents the first ionization of Mg and requires $I_1$=738 kJ/mol.
It is possible to continue removing electrons from the ion $Mg^+$ obtaining the $Mg^{2+}$. This second ionization always requires more energy than the first ($I_2$=1451 kJ/mol).
- Details
- Written by: Germán Fernández
- Category: periodic properties
- Hits: 1601
Electron affinity (EA) is defined as the energy exchanged (usually released) when an isolated ground-state gas atom picks up an electron to form an anion. Thus the F atom releases 328 kJ/mol when it captures an electron and becomes $F^-$. The fluoride anion is very stable because it has the electron configuration of neon.
\begin{equation} F(g)+1e^-\rightarrow F^-(g) \end{equation}
Although the atoms that release the most energy when capturing an electron are found on the periodic table on the right, it is observed that metals such as lithium, in a gaseous state, also release energy by forming lithium anions.
- Details
- Written by: Germán Fernández
- Category: periodic properties
- Hits: 1417
Electronegativity is defined as the ability of an element to attract to itself the electrons that link it with another element. This periodic property allows us to predict the polarity of the bond formed between two atoms, as well as its covalent or ionic nature.
Electronegativity is related to ionization energy and electron affinity. An atom with a very negative electron affinity and a high ionization potential has a high electronegativity (chlorine, fluorine). Conversely, atoms with low electron affinity and low ionization potential have low electronegativity (alkali).
Electronegativity therefore increases to the right and up the periodic table.
- Details
- Written by: Germán Fernández
- Category: periodic properties
- Hits: 1144
An atom or ion is diamagnetic if it has all its paired electrons. Diamagnetic substances are weakly repelled by magnetic fields.
An atom or ion is paramagnetic and has some unpaired electron. Paramagnetic substances are attracted to magnetic fields.



