Electronegativity is defined as the ability of an element to attract to itself the electrons that link it with another element. This periodic property allows us to predict the polarity of the bond formed between two atoms, as well as its covalent or ionic nature.
Electronegativity is related to ionization energy and electron affinity. An atom with a very negative electron affinity and a high ionization potential has a high electronegativity (chlorine, fluorine). Conversely, atoms with low electron affinity and low ionization potential have low electronegativity (alkali).
Electronegativity therefore increases to the right and up the periodic table.
A widely used electronegativities scale is the one created by Linus Pauling in 1904. The values of the Pauling scale oscillate between 0.7 for Francium and 4 for Fluorine. Using the table of electronegativities we are going to predict the polarity of some compounds.
- $F_2$. The electronegativity of fluorine is 4. The difference in electronegativity between the atoms that form the bond is: 4-4=0. It is a nonpolar covalent bond.
- H.F. The electronegativity of fluorine is 4, that of hydrogen 2.1. The electronegativity difference between both atoms is: 4-2.1=1.9. It is a polar covalent bond.
- LiF . The electronegativity of fluorine is 4, that of lithium 1. The difference in electronegativities: 4-1=3. It is a bond of such polarity that we call it ionic. In this bond, fluorine retains the bond's electrons and is in the form of a fluoride anion, while lithium is in the form of a lithium cation.
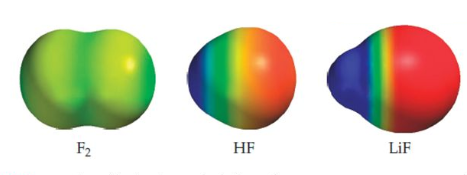
In this image we can observe the electronic densities in the $F_2$, HF and LiF molecules. The blue region is poor in electron density, while the red is rich.
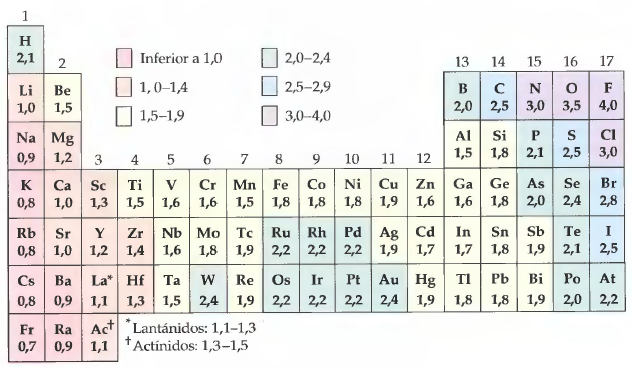
Pauling table of electronegativities.



