The chemical reactions
- Details
- Written by: Germán Fernández
- Category: chemical reactions
- Hits: 1151
Chemical reactions are very common in everyday life. When we turn on a gas stove, we are carrying out a combustion reaction, in which propane or butane gas combine with oxygen from the air to form carbon dioxide and water.
Another well-known reaction is the oxidation of Iron, its combination with oxygen from the air under appropriate conditions generates Iron oxide. This chemical reaction produces millions in losses, due to the rapid deterioration of tools, structures, etc.
There are very spectacular reactions, such as the one that takes place between Iron (III) oxide and aluminum metal (powdered), to produce liquid iron and aluminum oxide. This reaction gives off a large amount of heat, releasing products in the liquid phase. It is used to weld large iron structures and is known as thermite reaction.
- Details
- Written by: Germán Fernández
- Category: chemical reactions
- Hits: 1196
In a chemical reaction, a set of substances called reactants are transformed into others called products. A chemical transformation can be evidenced by the change in physical properties of the solution, such as: change in color, release or absorption of heat, formation of precipitates or release of gases.
To describe chemical reactions we use a notation, the chemical equation. The formulas of the reactants are represented on the left side of the chemical equation and those of the products on the right side, both separated by a full-headed arrow in the case of irreversible reactions (which only proceed from left to right) or a double arrow in the case of reversible reactions (they take place in both directions).
$$CH_4+O_2 \rightarrow CO_2 + H_2O$$
This chemical equation represents the combustion reaction of butane. Butane gas (CH 4 ) combines with oxygen (O 2 ) to irreversibly generate carbon dioxide (CO 2 ) and water (H 2 O)
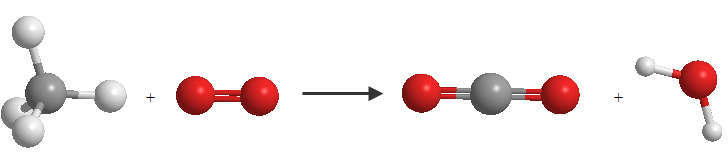
In this equation we see that there are different numbers of oxygen and hydrogen atoms on both sides of the equation, which requires an adjustment.
- Details
- Written by: Germán Fernández
- Category: chemical reactions
- Hits: 1221
In this type of adjustment we search for the stoichiometric coefficients that adjust the chemical equation. To simplify this operation we follow some simple guidelines.
- Elements that appear in only one reactant and product are matched first.
- Free elements fit last.
- The use of fractional coefficients can facilitate the adjustment. These fractional coefficients can be eliminated by multiplying the entire equation by the common denominator.
- Details
- Written by: Germán Fernández
- Category: chemical reactions
- Hits: 1230
Stoichiometry allows us to know the relationship between the amounts of compounds that participate in a chemical reaction. If we look at the stoichiometric coefficients of the adjusted for the synthesis of ammonia, \(N_2 + 3H_2 \rightarrow 2NH_3\), we can deduce that one molecule (or mol) of nitrogen reacts with three of hydrogen to form two of ammonia.
How many grams of ammonia will be obtained from 5.00 grams of nitrogen?
\begin{equation}\bbox[5px,border:2px solid red]{\color{red}{5.00\; g\; N_2\cdot \frac{1\;mol\; N_2}{ 28.01\;g\;N_2}\cdot \frac{2\;mol\;NH_3}{1\;mol\;N_2}\cdot\frac{17.00\;g\;NH_3} {1\;mol\;NH_3}=6.07\;g\;NH_3}}\end{equation}
Let's look at the hydrogen combustion reaction as an example.
\begin{equation} 2H_2(g)+O_2(g)\rightarrow H_2O(l) \end{equation}
- Details
- Written by: Jordi
- Category: chemical reactions
- Hits: 1233
For a chemist, expressions of the type:
$$\displaystyle CH_4 + 2\ O_2 \longrightarrow CO_2 + 2\ H_{2}O$$
They are intuitive and describe the global behavior of the substances that appear in them. This way of representing chemical reactions is simple and derives naturally from the use of chemical formulas as a written representation of the substances involved in any type of reaction. A chemist quickly identifies the name of the substance methane with its formula $CH_4$ and with everything that both representations, name and formula, mean: stoichiometric composition of the substance, molecular mass, type of chemical compound, physical characteristics... etc. . Years of individual study and the previous work of many generations of researchers hide all this behind a simple formula and a name. But on many occasions, it is necessary to convert this symbolism into something much more general. Mathematical calculations require the use of general expressions that can be treated numerically and that have a solid and rigorous notation system, a numerical formulation.
Let us consider a set made up of $S$ chemical compounds (it is understood that we are talking about pure substances in general, that is, both compounds in the strict sense, for example $CH_4$, and elements, $O_2$). We will designate each compound with a name: $A_1$, $A_2$, ..., $A_i$, ..., where $i$ $=$ $1$, $2$, ..., $S$. According to this form of representation, the reaction that we have mentioned before could be written:
$$\displaystyle CH_4 + 2\ O_2 \longrightarrow CO_2 + 2\ H_{2}O \quad\equiv\quad A_1 + 2\ A_2 = A_3 + 2\ A_4$$
where, in this case:
$$\displaystyle \begin{array}{c} S = 4\\ CH_4 \equiv A_1\\ O_2 \equiv A_2\\ CO_2 \equiv A_3\\ H_{2}O \equiv A_4 \end{array} $$
- Details
- Written by: Germán Fernández
- Category: chemical reactions
- Hits: 1180
At this point we will not limit ourselves to aqueous solutions, formed by water as a solvent and other minor components called solutes. Thus, when we write KCl(aq), we do not refer to an aqueous solution that contains KCl as a solute.
To work with these solutions, it is convenient to introduce new ways of establishing the ratio between solute and solvent, in addition to the percentage composition.
Molarity : It is defined as the ratio between the moles of solute and the volume of the solution. \begin{equation} Molarity\ (M)=\frac{Moles\ solute}{volume\ solution\acute{o}n\ (liters)} \end{equation} Molarity is represented by the symbol M and has units mol/L.
A 0.2 M (0.2 molar) solution of glucose contains 0.2 mol of glucose in 1 liter of solution.



