Chemical bonding I
- Details
- Written by: Germán Fernández
- Category: Chemical bond I
- Hits: 1308
Atoms tend to unite forming molecules to achieve electronic configurations of greater stability. There are two basic types of links:
- Ionic bonding results from the transfer of one or more electrons from the less electronegative atom to the more electronegative atom.
- The covalent bond results from the sharing of one or more electrons between the atoms that form the bond.
The ionic and covalent bonds represent theoretical extremes that do not usually occur in nature, the real bonds have both ionic and covalent character.
When the ionic character clearly predominates over the covalent, the compound is called ionic. In the case where the covalent character of the bond predominates, we speak of a covalent compound. Molecules formed by a single type of atom do not have an ionic character, they are pure covalent bonds (N 2 , O 2 , Cl 2 )
- Details
- Written by: Germán Fernández
- Category: Chemical bond I
- Hits: 1299
Ionic compounds have the following physical properties:
- They are solid with high melting points.
- They are soluble in polar solvents (water). However, they have low solubility in nonpolar solvents.
- Molten and in aqueous solution they conduct electric current.
- They are obtained from elements with different electronegativity (metal and non-metal)
Read more: Physical Properties of Ionic and Covalent Compounds
- Details
- Written by: Germán Fernández
- Category: Chemical bond I
- Hits: 1298
The electronic configuration of atoms is key to understanding how molecules are formed. Gilbert Newton Lewis concluded that atoms combine to achieve the electronic configuration of the noble gases.
Only the outermost electrons, called valence electrons, participate in the formation of a bond between two atoms. Lewis designed a system to represent the atom with its valence electrons, which consists of drawing the symbol of the element surrounded by dots, which represent each of the valence electrons.
Main ideas of Lewis's theory:
- Valence electrons are responsible for forming bonds.
- Electrons can be transferred between atoms giving rise to cations and anions that attract each other to form ionic compounds.
- When electrons are shared between atoms, covalent bonds are formed.
- The exchanged electrons allow the atoms to acquire the electronic structure of noble gas. They are generally surrounded by 8 electrons in their outer shell, called an octet.
- Details
- Written by: Germán Fernández
- Category: Chemical bond I
- Hits: 1344
Alkali metals tend to lose an electron to reach the noble gas electronic configuration, becoming positive ions. On the other hand, the halogens reach this configuration by adding an electron to their valence shell, becoming negative ions. The union of positive and negative ions by electrostatic forces generates a crystal lattice, an ionic compound.
Thus, lithium fluoride (LiF) is obtained when lithium (1s 2 2s 1 ) gives up one of its valence electrons to fluorine (1s 2 2s 2 2p 5 ), forming the lithium cation (1s 2 ) and the fluoride anion ( 1s 2 2s 2 2p 6 ). As can be seen in the electronic configurations, the lithium cation is isoelectronic to helium and the fluoride anion is isoelectronic to neon.
- Details
- Written by: Germán Fernández
- Category: Chemical bond I
- Hits: 1297
A covalent bond is formed when two atoms share one or more pairs of electrons. The condition for the bond to have a high covalent character is that the difference in electronegativity between the two atoms is zero or very small. According to the Lewis theory, the bond that gives rise to the hydrogen molecule can be described as follows: ![]()
- Details
- Written by: Germán Fernández
- Category: Chemical bond I
- Hits: 1357
The lattice energy gives us a measure of the stability of an ionic compound, and is defined as the energy required to completely separate one mole of a solid ionic compound into its ions in the gaseous state. When the ions unite to form the crystal lattice heat is given off (exothermic process), the ionic compound is more stable than the separated ions. To break the ionic compound separating the ions that form it, it is necessary to give an energy (equal to that given off when formed) called reticular.
The Born-Haber cycle allows obtaining the lattice energy of an ionic compound through the following stages, analyzed for the formation of LiF(s): 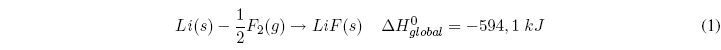
- Details
- Written by: Germán Fernández
- Category: Chemical bond I
- Hits: 1401
The following video shows how to build the Lewis structure of urea.
- Get the valence electrons: 24
- Choose the central atom: carbon
- Assign lone pairs to the terminal atoms.
- Complete the octet of the central atom
- Details
- Written by: Germán Fernández
- Category: Chemical bond I
- Hits: 1392
In this video I describe the steps to obtain the Lewis structure of thionyl chloride $(SOCl_2)$
- Calculation of valence electrons: 7x2+6+6=26
- draw the skeleton
- Complete the octet of the terminal atoms
- Give up electron pairs from terminal atoms to the central atom to minimize formal charge
- Details
- Written by: Germán Fernández
- Category: Chemical bond I
- Hits: 1288
Lewis structure of carbon dioxide, $(CO_2)$
- Valence electrons: 4+2x6=16
- Draw the skeleton, taking carbon as the central atom (less electronegative element)
- Fill in the octets of the terminal atoms (oxygens)
- Done lone pairs from the oxygens to complete the octet of the central atom (carbon)
- Details
- Written by: Germán Fernández
- Category: Chemical bond I
- Hits: 1246
In this video I describe the steps to follow to draw the Lewis structure of the nitrite ion, $(NO_2^-)$
- Details
- Written by: Germán Fernández
- Category: Chemical bond I
- Hits: 1426
To write the Lewis structure of the nitrosonium ion we follow the steps:
- Get the valence electrons: 5+6-1=10
- Draw the skeleton of the molecule.
- Distribute the valence electrons among the atoms, starting with oxygen.
- Give lone pairs to linkers to complete octets and look for the smallest formal charge.
- Details
- Written by: Germán Fernández
- Category: Chemical bond I
- Hits: 1239
The following video describes the construction of the structure of the triiodide ion $(I_3^-)$.
- Details
- Written by: Germán Fernández
- Category: Chemical bond I
- Hits: 1214
In this video the Lewis structure of xenon oxytrifluoride is obtained. In addition, formal charges are assigned to determine the correct structure of the molecule.
- Details
- Written by: Germán Fernández
- Category: Chemical bond I
- Hits: 1214
In the following video we build the Lewis structure of xenon tetrafluoride. The steps to follow are:
- Calculate valence electrons: 8+7x4=36
- Write the molecular skeleton with the xenon in the center.
- Fill in the octets of the terminal atoms (fluorine)
- Place the remaining pairs on the central atom
- Assign formal charges and cede lone pairs to binders if necessary.
- Details
- Written by: Germán Fernández
- Category: Chemical bond I
- Hits: 1301
The following video shows how to write the Lewis structure of hydrocyanic acid, HCN.
- Get the valence electrons 4+5+1=10
- draw the skeleton of the molecule
- Assign lone electrons to terminal atoms completing their octet
- Give lone pairs to linkers to complete the octet of the central atom.
- Assign formal charges to see that the structure drawn is the most probable.
- Details
- Written by: Germán Fernández
- Category: Chemical bond I
- Hits: 1414
In this video I discuss the necessary steps to write the Lewis structure of sulfuric acid $(H_2SO_4)$:
- Calculate the number of valence electrons: 6x4+6+2=32
- Draw the molecular skeleton.
- Complete octet of terminal atoms.
- Give lone pairs to bonding pairs to find the structure that minimizes the formal charge of the atoms.
- Details
- Written by: Germán Fernández
- Category: Chemical bond I
- Hits: 1215
In this video the Lewis structures of carbonic acid, phosphoric acid and phosphorous acid are built. Special attention should be paid to the peculiarity of phosphorous acid, which only has two acidic hydrogens.



