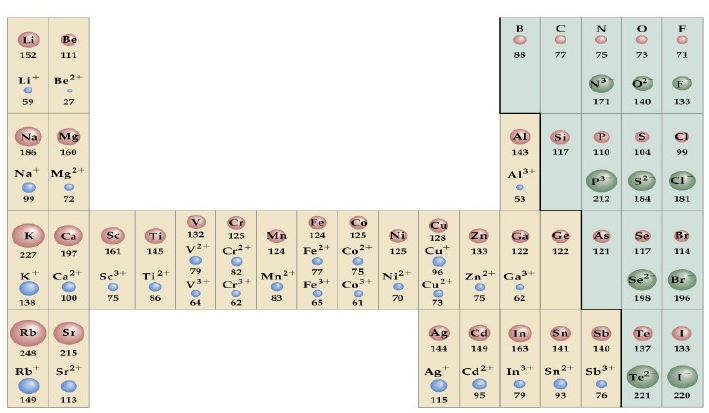
Since the electron density of an atom never goes to zero it is never difficult to define the size of atoms. One possibility is to consider the radius of the sphere that contains a high probability of finding the electron, for example 90$\%$. However, we are more interested in knowing the size of the atoms when they are linked, forming covalent or ionic compounds.
The covalent radius is defined as half the distance between the nuclei of two identical atoms joined by a single covalent bond. The ionic radius is the distance between the nuclei of two atoms joined by an ionic bond. In the latter case, the distance must be distributed correctly between the cation (smaller) and the anion (larger).
- Increase in atomic radius in a group . Increasing the value of n shifts the probability density toward higher values of r, giving rise to a larger atom. A significant increase in radius is observed when passing from Li to Na and from Na to K. The increase in radius in elements with high atomic number (passage from potassium to rubidium) is less due to the existence of d and f subshells that shield the nucleus with less effective than the syp, causing the outer electrons to be more attracted to the nucleus.
- Decrease in atomic radius in a period . Moving to the right in one period produces an increase in the effective nuclear charge that acts on the outer electrons, compressing the atom. In the particular case of the transition elements, this variation is small because the electrons gradually enter an internal (n-1)d subshell that contributes to the shielding of the external electrons, ns.
Regarding the ionic radius, it is true that:
- Cations are smaller than the atom from which they came . The cation is formed by the loss of one or more outer electrons, the rest being more attracted to the nucleus.
- Anions are larger than the atom from which they came . Anions are formed when the neutral atom captures one or more electrons. This electronic excess produces a decrease in the effective nuclear charge, which translates into a lower attraction of the external electrons by the nucleus.
- Isoelectronic cations are smaller the larger their charge, while isoelectronic anions are larger the larger their charge.