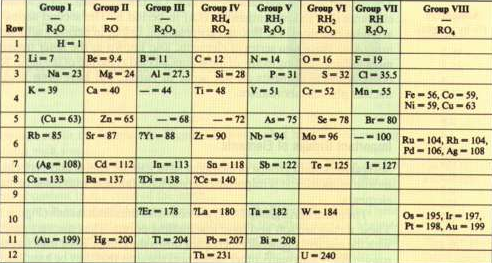
Mendeleev arranged the elements according to atomic masses, leaving blank spaces for elements yet to be discovered. Thus he predicted the existence of the elements of atomic mass 44, 68, 72 and 100 corresponding to scandium, gallium, germanium and technetium.
Elements with similar properties were grouped into columns (groups), their physical properties gradually changing as we went down the group.
Mendeleev's periodic table is missing the group of noble gases, discovered by William Ramsay and placed in a separate group of the table.