I am going to illustrate how to determine the specific heat of a substance, through a very visual procedure, exposed by Ralph Petrucci in his book "General Chemistry".
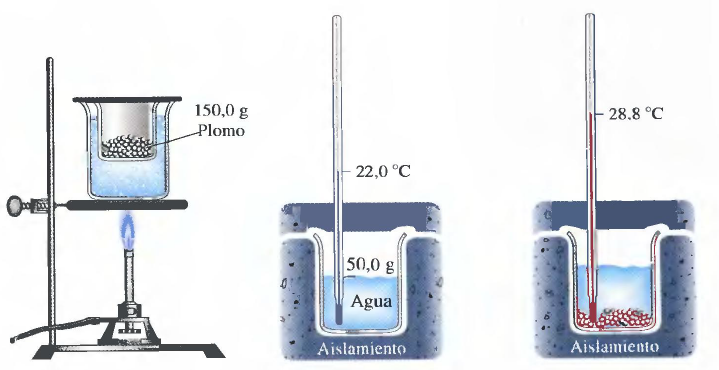
The substance of which we will determine its specific heat is lead. The first step is to heat the lead to a known temperature. A very simple way is to heat it in a water bath, bringing the water to a boil, which guarantees a temperature of 100ºC.
Next, we introduce water and a thermometer to measure its temperature in a thermally insulated glass (covered with polystyrene).
The last step consists of mixing the lead at 100ºC with the water at 22ºC, waiting for both substances to reach thermal equilibrium and measuring the final temperature.
Now we apply the principle of conservation of energy to determine the specific heat of lead:
$Q_{ceded\;lead}=-Q_{gained\;water}$



