 By the early 1900s chemists knew that the atom contained negatively charged particles (electrons) and positive particles (protons), the only remaining question was how to distribute them in the atom.
By the early 1900s chemists knew that the atom contained negatively charged particles (electrons) and positive particles (protons), the only remaining question was how to distribute them in the atom.
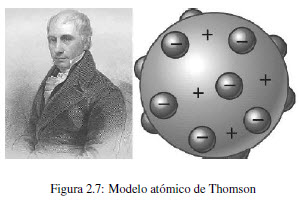
JJ Thomson proposed an atomic model that consisted of uniformly distributing negative particles within a positively charged sphere. This model was called "raisin pudding" because of its resemblance to the famous English dessert.



