The Greek philosopher Democritus, in the 5th century BC, hypothesized that matter was made up of small, indivisible particles which he called atoms.
In 1808, the English scientist, John Dalton, took up the ideas of Democritus, formulating them in a more precise way. The hypotheses on which Dalton's theory is based can be summarized in three points:
- Elements are made up of very small particles called atoms. The atoms of an element are identical (same mass, chemical properties) but different from the atoms of other elements.
- Compounds are formed by the union of atoms of two or more elements. The ratio between the number of atoms present in a compound is always a whole number or a simple fraction.
- In chemical reactions, atoms are separated, combined, or regrouped, never created or destroyed.
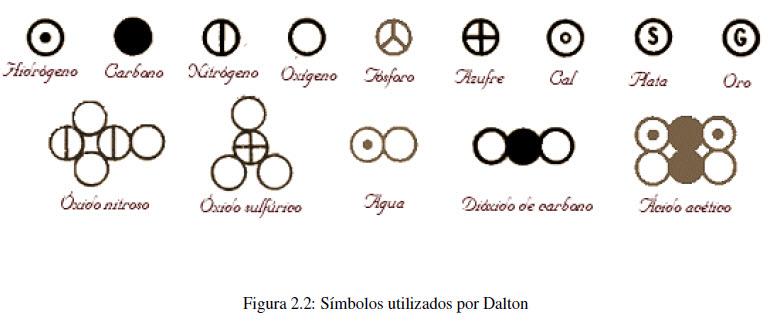
In the first hypothesis, Dalton establishes that the different properties shown by the chemical elements are due to the fact that the atoms that compose them are different. Thus, hydrogen and oxygen are elements that have different properties because the atoms that constitute them are different.
The second hypothesis allows us to explain the Law of definite proportions, enunciated by Proust in 1799, which establishes that different samples of the same compound always contain the same elements and in the same proportion by mass. Since compounds are formed by combining atoms of elements in a simple ratio, regardless of the origin of the compound, the same ratio by mass will always be found.
In addition, the second hypothesis allows to explain the law of multiple proportions. According to this law, if two elements can combine to form more than one compound, the mass of one element combining with a fixed mass of the other maintains a ratio of small whole numbers. Different compounds made up of the same elements differ in the number of atoms of each kind.
Dalton's third hypothesis, indestructibility of the atom, is an alternative statement to the law of conservation of mass, which states that matter is neither created nor destroyed in chemical processes.



