The periodic table arranges the elements by increasing atomic number, starting at the top left. Elements located in the same column (group) have similar properties. For example, group 1 includes the alkali metals (lithium, sodium, potassium, rubidium and cesium), all of which are good conductors of heat and electricity, and have a violent reaction with water. Group 2 is made up of the alkaline earth metals (beryllium, magnesium, calcium, strontium, barium, and radium). The elements of group 17 are called halogens (fluorine, chlorine, bromine and iodine) and those of group 18 noble gases (helium, neon, argon, krypton, xenon and radon).
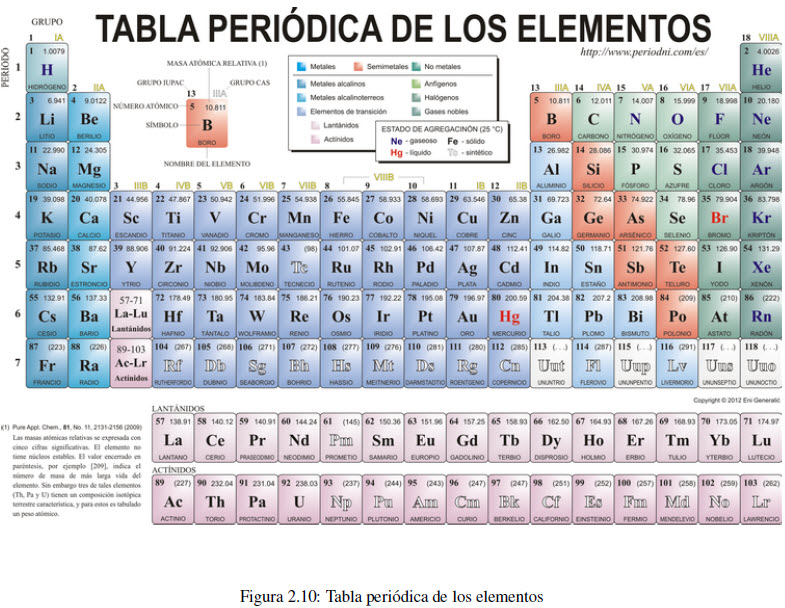
It is customary to divide the elements into two broad categories called metals and non-metals. At room temperature all metals are solid (with the exception of mercury), are good conductors of heat and electricity, and can be drawn into sheets and wires, properties that are known as malleable and ductile.
Non-metals are located on the right side of the periodic table, some are gases (oxygen, nitrogen, chlorine), bromine is liquid, and solids are highly brittle (sulfur, phosphorus).
Between both groups there is a small number of elements with intermediate properties, called metalloids (boron, silicon, germanium, arsenic, antimony, tellurium, polonium and astatine).



