AV constant the work done is null \(w=0\).
Therefore, \(\Delta U=q\)
The heat is calculated with the relationship:
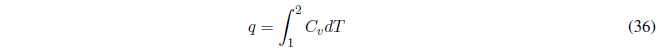
If the heat capacity is constant, the integral gives us:
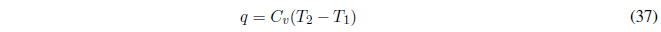
The calculation of the enthalpy change is done with: \(\Delta H=\Delta U + V\Delta P\)



