The enthalpy H of a thermodynamic system is defined as: ![]()
The importance of this magnitude in chemistry lies in the fact that its variation coincides with the heat exchanged at constant pressure. It is very common for a chemical reaction to take place in an open vessel (at atmospheric pressure) and the heat exchanged will be an enthalpy change.
We start from the first law of thermodynamics: ![]() , the subscript p indicates constant pressure.
, the subscript p indicates constant pressure. 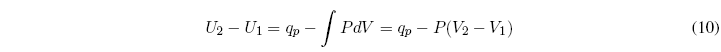
Clearing the heat and grouping terms we obtain: 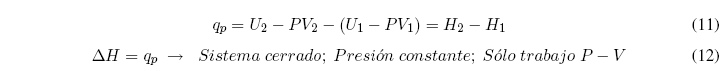
Now let's see what happens when the volume of the system remains constant: 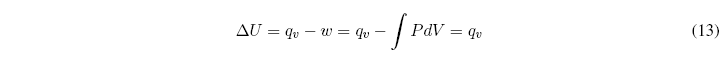
At constant volume the work is zero and the heat exchanged at constant volume coincides with the change in internal energy. ![]()
In any chemical process that maintains the constant volume, the heat exchanged coincides with the change in internal energy of the system.



