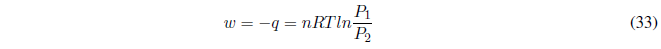During this process the temperature is kept constant. Since the internal energy of ideal gases only depends on temperature: \(\Delta U=0\) and \(\Delta H=0\).
The first principle tells us: \(\Delta U=q+w\;\;\rightarrow q=-w\) . In this process, the heat exchanged is equal to the work done. We calculate the work done by the gas using the equation of state.
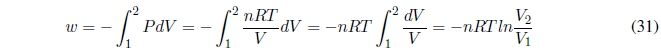
It can be put as a function of the initial and final state pressures using Boyle's law,
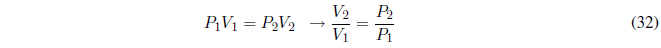
Substituting: