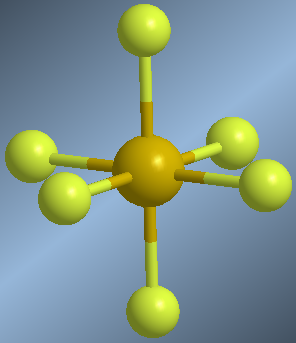
 Molecules whose central atom is surrounded by 6 bonding pairs are arranged with octahedral geometry. This spatial arrangement allows minimizing the repulsions between the 6 bonding pairs and is equivalent to a square dipyramid. Atoms located in an equatorial position form 90º angles with each other. Atoms in axial position also form angles of 90º with respect to the equatorial ones and 180º between them.
Molecules whose central atom is surrounded by 6 bonding pairs are arranged with octahedral geometry. This spatial arrangement allows minimizing the repulsions between the 6 bonding pairs and is equivalent to a square dipyramid. Atoms located in an equatorial position form 90º angles with each other. Atoms in axial position also form angles of 90º with respect to the equatorial ones and 180º between them.
An example of this geometry occurs in sulfur hexafluoride, SF 6 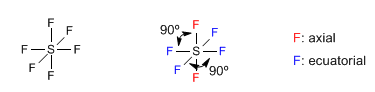
Chemical bonding II
Molecules with Octahedral Geometry, AB6
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1828



