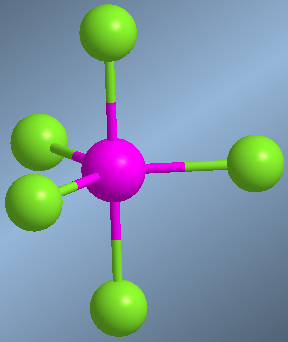
 When a central atom joins 5 groups, the spatial arrangement that minimizes the repulsions between the bonding pairs is the trigonal dipyramid.
When a central atom joins 5 groups, the spatial arrangement that minimizes the repulsions between the bonding pairs is the trigonal dipyramid.
Let's look at the example of phosphorous pentachloride, PCl 5 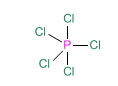
The atoms located in the triangular plane are called equatorial and those above and below this plane, axial. Equatorial chlorines have bond angles of 120º. Between an axial and an equatorial chlorine the angle is 90º.
Chemical bonding II
Trigonal dipyramid, AB5
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1389



