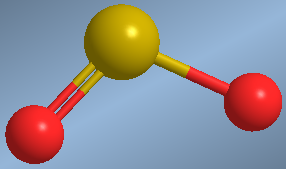
 Molecules that have a lone pair on the central atom and two bonding pairs have an angular geometry.
Molecules that have a lone pair on the central atom and two bonding pairs have an angular geometry.
An example of this geometry is found in sulfur dioxide, SO 2 
The bond angle between the oxygens is less than 120º due to the strong repulsion with the lone pair. 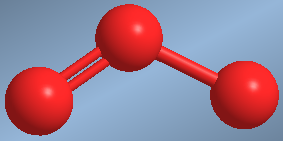 A second example of angular geometry is found in ozone, O 3
A second example of angular geometry is found in ozone, O 3 



