They are molecules with two binding pairs and two lone pairs. These four groups are arranged towards the vertices of a 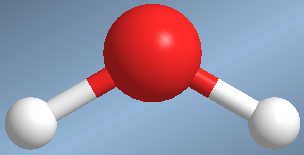 tetrahedron. However, to give the geometry only bonding pairs (not lone pairs) are taken into account and therefore the geometry of these molecules is angular.
tetrahedron. However, to give the geometry only bonding pairs (not lone pairs) are taken into account and therefore the geometry of these molecules is angular.
An example of this type is found in water, H 2 O 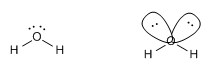
The HOH angle is 104.5º, due to the repulsion of the lone pairs on the bonding pairs.
Chemical bonding II
Angular Molecules, AB2E2
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1980



