They are molecules that have three binding pairs and a lone pair surrounding a central atom. The spatial arrangement 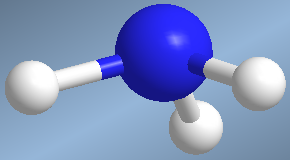 of these four pairs is tetrahedral, however, by neglecting the lone pair, we are left with a pyramidal geometry.
of these four pairs is tetrahedral, however, by neglecting the lone pair, we are left with a pyramidal geometry.
One such molecule is ammonia, NH 3 
The lone pair strongly repels the bonders and the HNH bond angle is less than 109°.
Chemical bonding II
Molecules with pyramidal geometry, AB3E
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1376



