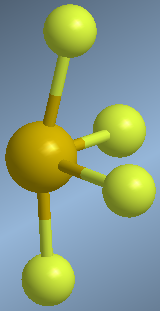
 Molecules with four binding pairs and one lone pair are arranged in the form of a trigonal dipyramid. However, removing the lone pair results in a kind of distorted tetrahedron.
Molecules with four binding pairs and one lone pair are arranged in the form of a trigonal dipyramid. However, removing the lone pair results in a kind of distorted tetrahedron.
One molecule that exhibits this spatial distribution of groups is sulfur tetrafluoride, SF 4 . 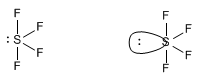
Observe how in the molecular model the axial sulfurs are bent to the right, due to the repulsions with the lone pair.
In the proposed geometry, the para solitaire occupies the equatorial position, undergoing repulsions with two bonding pairs (axial) that are at 90º. If we had placed the lone pair in an axial position, the interactions would have been with three bonding pairs at 90º. 
Chemical bonding II
Distorted tetrahedral geometry, AB4E
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 1963



