Molecules with three binding pairs and two lone pairs are arranged in a T-shape. The geometry that minimizes the 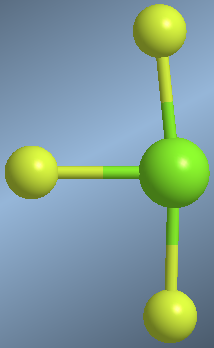 repulsions is the one that arranges the lone pairs in equatorial. Considering the lone pairs, the geometry is a trigonal dipyramid and removing the lone pairs gives us the T shape.
repulsions is the one that arranges the lone pairs in equatorial. Considering the lone pairs, the geometry is a trigonal dipyramid and removing the lone pairs gives us the T shape.
A molecule that responds to this geometry is chlorine trifluoride, ClF 3

As can be seen in the model, the axial fluorines bend slightly to the right due to the repulsions with the lone pairs.
By removing the lone pairs from the drawing, the trigonal dipyramid becomes a T.
Chemical bonding II
Molecules with T-shaped geometry, AB3E2
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 2166



