Molecules with two binding pairs and three lone pairs are arranged in a trigonal bipyramidal shape with the three pairs ![]() lonely in equatorial Without taking into account the lone pairs we obtain a linear geometry.
lonely in equatorial Without taking into account the lone pairs we obtain a linear geometry.
An example of this geometry is found in the triiodide ion, I 3 - 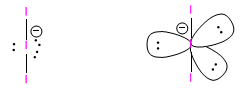
The location of the lone pairs in equatorial minimizes the repulsions between them, as they are located at 120º.
Chemical bonding II
Molecules with linear geometry, AB2E3
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 2140



