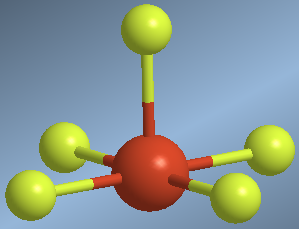
 They are molecules with five binding pairs and one solitary pair. The spatial layout including the lone pair is octahedral, without considering the lone pair we are left with a square pyramid.
They are molecules with five binding pairs and one solitary pair. The spatial layout including the lone pair is octahedral, without considering the lone pair we are left with a square pyramid.
An example of this geometry is found in the bromine pentafluoride molecule, BrF 5 
Chemical bonding II
Molecules with square pyramidal geometry, AB5E
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 2380



