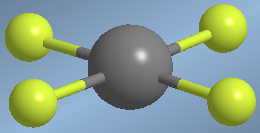
 Molecules with four bonding pairs and two lone pairs present an octahedral spatial arrangement that becomes a square plane when lone pairs are not considered.
Molecules with four bonding pairs and two lone pairs present an octahedral spatial arrangement that becomes a square plane when lone pairs are not considered.
An example of this spatial arrangement is found in xenon tetrafluoride, XeF 4 
Chemical bonding II
Molecules with square plane geometry, AB4E2
- Details
- Written by: Germán Fernández
- Category: chemical bond II
- Hits: 3403



