They are AB 2 type molecules in which the central atom has no lone pairs. To avoid repulsions between the bonding pairs, arrange the B groups as far apart as possible. 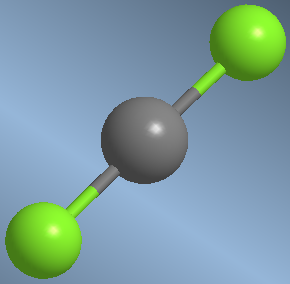 An example is beryllium dichloride, BeCl 2 .
An example is beryllium dichloride, BeCl 2 . ![]()
The molecule is linear with bond angles of 180º.
A second example is the carbon dioxide molecule, CO 2 ![]()
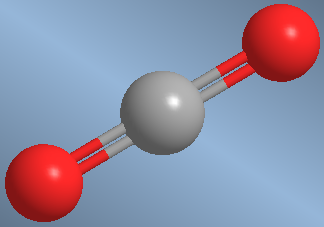 Carbon dioxide arranges the C=O bonds at 180º to avoid repulsions.
Carbon dioxide arranges the C=O bonds at 180º to avoid repulsions.



