The Lewis theory and the VSEPR model have given us a fairly accurate description of the structure and geometry of the molecule. ![]()
Now we will try to better understand the formation of Be-Cl bonds using the valence bond theory. For this we will write the electronic configuration of beryllium, looking at the valence layer. 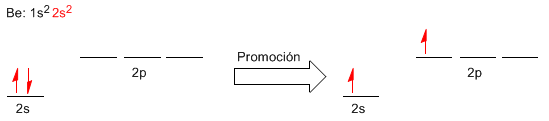
Electron promotion involves the passage of an electron from the 2s level to the 2p level of higher energy. The promotion allows having two orbitals available to form bonds. We could explain the two bonds formed in BeCl 2 by overlapping of the 3p orbitals of the chlorines with the 2s and 2p orbitals of the beryllium. However, the bonds formed in BeCl 2 are the same (same length and energy), properties that we cannot explain if we form these bonds from different atomic orbitals.

Let's see the hybridization process in a more graphic way 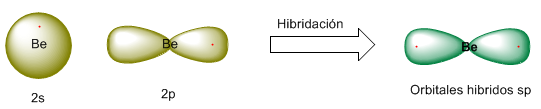
Each of the two hybrid orbitals contains an electron that, when overlapping with 3p orbitals of the chlorine atom, forms Be-Cl bonds. 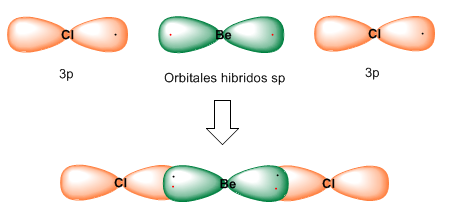
sp hybridization gives rise to linear molecules with bond angles of 180º.



