The rate of a reaction can strongly depend on the solvent. As an example, consider $S_N2$: $CH_3I + Cl^- \rightarrow CH_3Cl + I^-$, the speed depends on the solvent since this changes the value of k, due to the different solvation of the reactants. At other times, very fast reactions are limited by the rate of diffusion of the reactants through the solvent (solvent viscosity). The value of k also depends on hydrogen bonding. A reaction that can proceed through two competing mechanisms, rates can be affected and follow one mechanism in one solvent and the other in a different solvent.
Encounters, collisions, and the cell effect
In a gas the molecules are far apart and move freely. In a liquid, a molecule is surrounded by other molecules "solvent cell". This molecule crashes into the cell walls before being able to escape.
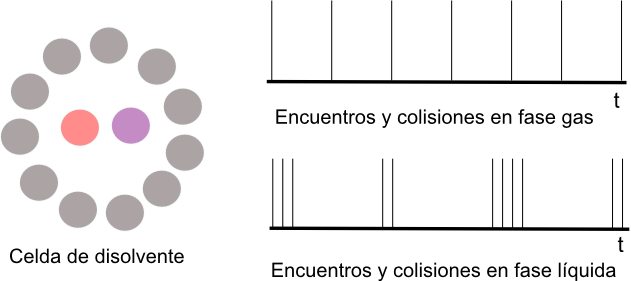
When two reactive molecules A and B are in solution they must diffuse through the solvent in order to meet. However, once they meet, they will be surrounded by the solvent that will keep them close for a long time, colliding many times before leaving the cell, which favors their reaction. When A and B are in the same cell, it is called an encounter. Each collision is called a collision.
Diffusion-controlled reactions
Suppose that the reaction $A + B \rightarrow C$, has a low activation energy, therefore there is a high probability for the reaction to occur in each collision. Since many collisions occur in each meeting, it is very likely that a reaction will occur each time A and B meet. The number of encounters depends on the rate with which A and B diffuse into the solution. These reactions have a rate controlled by diffusion.



