 In 1924, the Frenchman Victor de Broglie published his doctoral thesis, in which he developed the hypothesis that matter particles could exhibit wave behavior. De Broglie was inspired by Einstein's work on the photoelectric effect, in which light was made up of small corpuscles called photons, whose energy was $h\nu$. If a clearly wave-like phenomenon such as light had this corpuscular behavior, it might be possible for matter particles to have wave behavior.
In 1924, the Frenchman Victor de Broglie published his doctoral thesis, in which he developed the hypothesis that matter particles could exhibit wave behavior. De Broglie was inspired by Einstein's work on the photoelectric effect, in which light was made up of small corpuscles called photons, whose energy was $h\nu$. If a clearly wave-like phenomenon such as light had this corpuscular behavior, it might be possible for matter particles to have wave behavior.
Equating Einstein's equation, $E=mc^2$ to Planck's, $E=h\nu$:
$mc^2=h\nu$, replacing the frequency with $\nu=c/\lambda$:
$mc^2=h\frac{c}{\lambda}$, solving for the wavelength:
\begin{equation}\lambda=\frac{h}{mc}=\frac{h}{p}\end{equation}
Being p the quantity of movement or linear momentum of the particle.
This equation tells us that every particle that has momentum, photon, electron, proton, has an associated wave of length $\lambda$.

Even macroscopic particles, like a tennis ball, have this wave behavior. Although since the mass of these particles is large, the associated wavelength is very small, and no type of wave behavior is observed.
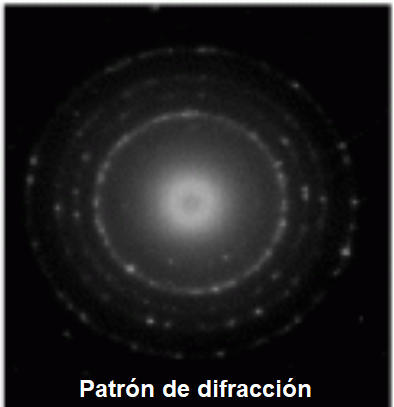 In atomic particles, this wave can be much larger than the size of the particle, giving it a clearly wavelike behavior, giving rise to phenomena such as diffraction.
In atomic particles, this wave can be much larger than the size of the particle, giving it a clearly wavelike behavior, giving rise to phenomena such as diffraction.
In 1927, Davisson and Germer obtained a diffraction pattern by passing electrons through a crystal lattice, showing that the electron behaved like a wave.
From now on, when we imagine an atom, we should not see electrons as particles revolving in defined orbits around the nucleus. In reality, electrons behave like matter waves, without a precise location, the wave extending throughout all space.



