It is a physical phenomenon that consists of the emission of electrons by certain metals when a beam of light falls on its surface. It was observed in 1888 by Heinrich Hertz.
The photoelectric effect is characterized by:
- For the emission of electrons to occur, the incident light must have a minimum frequency, called the threshold frequency $\nu_0$
- The number of emitted electrons depends on the intensity of the incident radiation.
- The kinetic energy of electrons depends on the frequency of light.
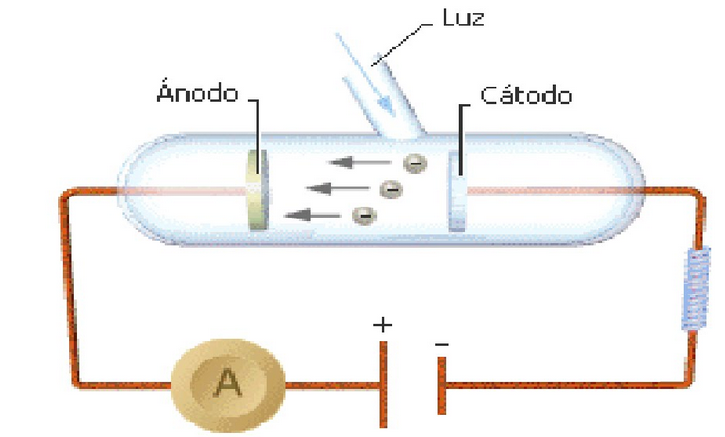
The classical theory could not explain that the photoelectric effect depended on the frequency of the radiation. However, Einstein, in 1905, proposed that electromagnetic radiation was made up of light particles, called photons, whose energy is given by Planck's equation, $E=h\nu$, dependent on the frequency of the radiation.
The photoelectric effect occurs when a photon collides with an electron and removes it from the metal, the photon must have a minimum energy ($h\nu_0$) equal to the ionization energy of the atom, so that the electron can leave the influence of the core. If we use lower energy radiation (frequency) the photoelectric effect is not observed. the intensity of the radiation is related to the number of photons that fall on the metal, the greater the number of photons, the greater the number of emitted electrons.
The energy of the photon is used to remove the electron and supply it with kinetic energy, and the following energy balance can be written:
\begin{equation} E_{photo\acute{o}n}=E_{ionization} +E_{kin\acute{e}tics} \end{equation} Substituting values: \begin{equation} h\nu=h\nu_0+\frac{1}{2}mv^2 \end{equation}



