Spin is an intrinsic property of the electron that was initially associated with rotation, but since it does not depend on spatial coordinates it is independent of the state of motion of the particle. The value of the spin angular momentum is quantized, depending on a quantum number, $m_s$, which takes values +1/2 and -1/2.
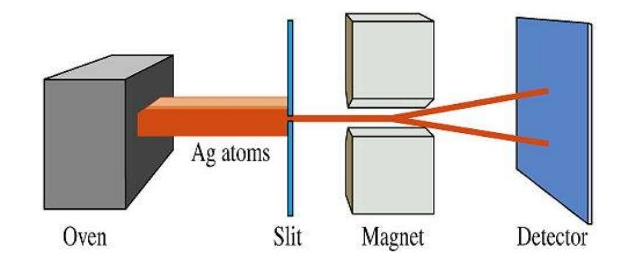
The experimental demonstration of the existence of spin was carried out by Stern and Gerlach in 1920. By subjecting a beam of silver atoms, vaporized in an oven, to a variable magnetic field, they observed the splitting of the beam into two beams, due to the presence of an unpaired electron that in half of the silver atoms has $m_s=1/2$ and in the other half $m_s=-1/2$.