As we saw in previous sections, the energy levels of the hydrogen atom are given by the principal quantum number (n), being independent of the quantum numbers l and m. This situation changes in atoms with more than one electron due to the interaction between them. In polyelectronic atoms, the energy of the electron also depends on the quantum number l, with the electrons in the 2s subshell and those in the 2p being located at different energy levels. In the same way, the 3s, 3p and 3d have different energy.
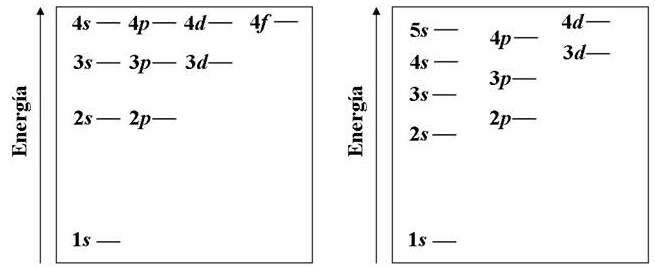
The diagram on the left represents the energy levels of hydrogen atoms (one electron). On the right we have the energy levels for a many electron atom.



