It is a reversible cycle that consists of two isothermal stages at different temperatures and two adiabatic stages. The working substance may not be an ideal gas, but in this development for simplicity we will use one mole of ideal gas.
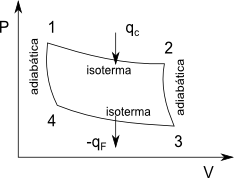
The first stage of the cycle is an isothermal reversible expansion (line 1-2). In this step, a quantity of heat is absorbed from the hot source. The second stage is an adiabatic expansion from the temperature of the hot source to the temperature of the cold source (line 2-3). The third stage is an isothermal compression that gives up a quantity of heat to the cold source (lines 3-4). The last stage is the adiabatic compression that brings the system back to the starting point (line 4-1).



