In the classical world we can accurately measure the momentum and position of a particle. However, in the quantum world 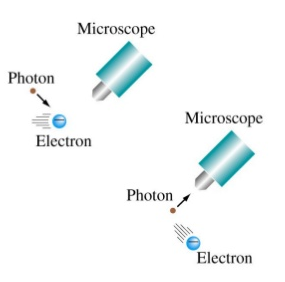 these magnitudes are affected by an uncertainty that prevents knowing their values simultaneously. Knowledge of the position of the particle produces a lack of knowledge of its momentum or velocity. Calling $\Delta x$ the uncertainty in the position and $\Delta p$ the uncertainty in its momentum, we can write the expression of the uncertainty principle as follows:
these magnitudes are affected by an uncertainty that prevents knowing their values simultaneously. Knowledge of the position of the particle produces a lack of knowledge of its momentum or velocity. Calling $\Delta x$ the uncertainty in the position and $\Delta p$ the uncertainty in its momentum, we can write the expression of the uncertainty principle as follows:
\begin{equation} \Delta x \cdot \Delta p\geq \frac{h}{4\pi} \end{equation}
To better understand this idea, let's imagine that we are interested in observing the electron in the hydrogen atom. To do this, we must send a photon, of the appropriate wavelength, that hits the electron and returns to our eyes. The problem is that said photon is so energetic that it will change the state in which said electron is found, even going so far as to ionize the atom. In short, we cannot observe quantum particles without modifying them. Obviously macroscopic objects do not present this problem, bombarding a tennis ball with photons does not change its state of motion.
Example. What is the uncertainty in the velocity of a proton beam whose position is known to the uncertainty of 24 nm?
Solution:
\begin{equation} \Delta x \cdot \Delta p\geq\frac{h}{4\pi} \end{equation}
Where, $\Delta p=m\Delta v$. Clearing $\Delta v$:
\begin{equation} \Delta v\geq \frac{h}{4\pi m\Delta x}=\frac{6.626x10^{-34}Js}{4\pi 1.67x10^{-27}kg 2.4 x10^{-8}m}=1.3\ m/s \end{equation}



