Charles in 1787 and Gay-Lussac in 1802 studied the thermal expansion of gases and found a proportionality between volume and temperature, called Charles Gay-Lussac's law.
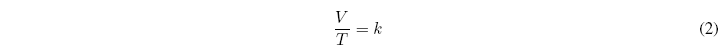
For a given mass of gas and constant pressure it is true that the ratio of the volume occupied by a gas and the temperature at which it is found is constant. This law gives fairly accurate results under low pressure conditions.
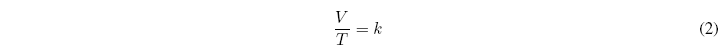
For a given mass of gas and constant pressure it is true that the ratio of the volume occupied by a gas and the temperature at which it is found is constant. This law gives fairly accurate results under low pressure conditions.



