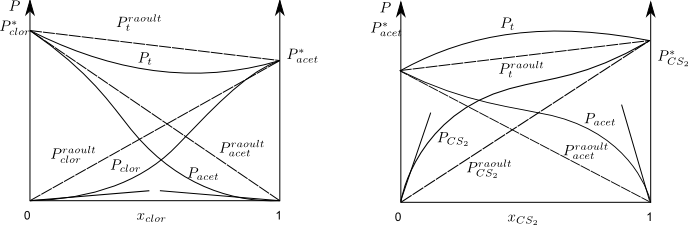
Negative deviations from Raoult's Law.
The acetone-chloroform solution has vapor pressures lower than those predicted by Raoult's Law. This deviation is due to the fact that the intermolecular forces are greater in the solution than in the pure components.
As can be seen from the graph for chloroform mole fractions close to 1, chloroform follows Raoult's Law while acetone follows Henry's Law.
For chloroform mole fractions close to 0, acetone follows Raoult's Law and chloroform follows Henry's.
Positive deviations from Raoult
The acetone-carbon disulfide solution presents vapor pressures higher than those predicted by Raoult, due to the fact that the intermolecular forces in the solution are less than in the pure components.