Let us consider a system with only one component $(H_2O)$, we neglect the dissociation. We are going to represent any state of the system by means of a phase diagram.
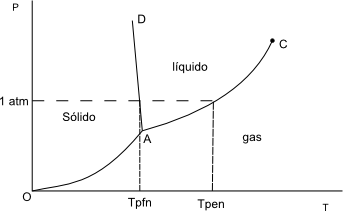
At low temperatures and moderate or high pressures we have the solid phase. At low pressures the gas phase and at intermediate temperatures and pressures the liquid phase.
- OA: Solid-gas phase equilibrium, gives us the sublimation points of the solid.
- AD: Solid-liquid balance, gives us the melting points.
- AC: Liquid-gas equilibrium, gives us the boiling points.
- A: triple point. Point at which the three phases coexist: solid, liquid and gas.
- C: Critical point. Above $T_c$ it is not possible to liquefy a gas by compression.
Fixed the pressure at 1 atm, the cutoff points with the AD and AC lines give us the normal melting and normal boiling points (0ºC and 100ºC for water).
At the critical point the density of the liquid and vapor phases are equal, liquid and gas have the same properties.



