 Inorganic chemistry is the branch of chemistry that studies the properties, structure, and reactivity of inorganic compounds.
Inorganic chemistry is the branch of chemistry that studies the properties, structure, and reactivity of inorganic compounds.
This field of chemistry covers all chemical compounds, excluding those with carbon-hydrogen bonds, which are the object of study by organic chemistry.
Both disciplines share numerous points in common, and interdisciplinary fields of great importance are emerging, among which we can mention organometallic chemistry.
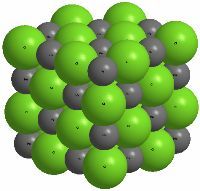
The most important part of inorganic compounds are formed by the combination of cations and anions linked by ionic bonds. Thus, NaCl is formed by the union of sodium cations with chloride anions. The ease with which an ionic compound is formed depends on the ionization potential (for the cation) and the electron affinity (for the anion) of the elements that generate the respective ions.
The most important inorganic compounds are oxides, carbonates, sulfates, ect. Most inorganic compounds are characterized by high melting points, low conductivity in the solid state, and high solubility in aqueous media.
At an industrial level, inorganic chemistry is of great importance. It is customary to measure the development of a nation by its productivity in sulfuric acid. Among the most manufactured chemical products worldwide, it is worth mentioning ammonium sulfate, ammonia, ammonium nitrate, ammonium sulfate, hypochlorous acid, hydrogen peroxide, nitric acid, nitrogen, oxygen, sodium carbonate......
Physical chemistry
Definition of Inorganic Chemistry
- Details
- Written by: Germán Fernández
- Category: Start
- Hits: 1713



