Classical thermodynamics studies the equilibrium properties of systems, the processes being reversible. Now we are going to study systems that are out of equilibrium, in which irreversible processes occur. A system is out of equilibrium because matter or energy is transported between the system and the surroundings, or between different parts of the system. These out-of-equilibrium systems evolve, over time, following irreversible processes towards equilibrium states.
Two examples of irreversible processes are the dissolution of a salt and the transfer of heat between two heat sources at different temperatures. 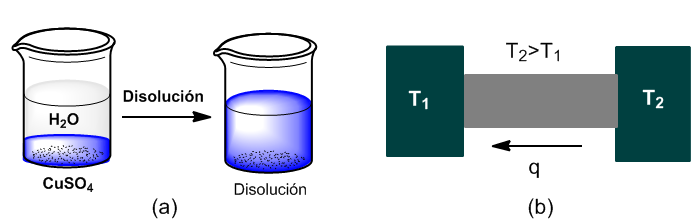
In scheme (a) we have a system formed by two phases (copper sulfate and water), which evolves irreversibly until the total dissolution of the salt or saturation of the solution.
In diagram (b) two heat sources in contact evolve until they reach thermal equilibrium.



