The solubility of a salt decreases when a certain concentration of any of the ions that the salt generates upon dissolution (common ions) previously exists in the medium. The solubility of lead iodide in pure water at 25°C is $1.2x10 -3 $mol of lead iodide per liter of solution. If previously in the water we dissolve a certain amount of potassium iodide and then add lead iodide, we will observe a drastic decrease in its solubility, since iodide is a common ion. In the same way, if we dissolve lead iodide in water that contains certain amounts of lead, the solubility of the salt will be reduced by the presence of the common ion (in this case $Pb^{2+}$) We can also appreciate the effect of the common ion by adding to a saturated solution of lead iodide certain quantities of sodium iodide that will immediately precipitate the lead iodide. The explanation for this phenomenon lies in Le Châtelier's principle, according to which adding a product of a reaction in equilibrium produces a displacement towards the reactants. Adding iodide anions or lead cations shifts the solubility equilibrium towards the solid ($PbI_2$).
Calculate the molar solubility of lead iodide in a 0.1 M KI aqueous solution. $K_{ps}(PbI_2)=7.1x10^{-7}$
Solution:
We write the solubility equilibrium equation for lead iodide and put the initial and equilibrium molar concentrations below.
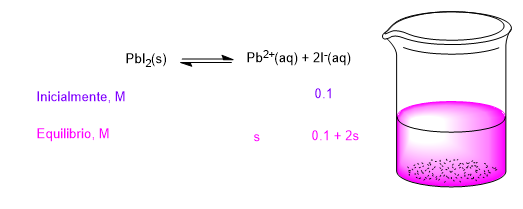
Taking these equilibrium concentrations to the solubility product constant:
\begin{equation} K_{ps}=[Pb^{2+}][I^-]^2=s(0.1+2s)^2=7.1x10^{-9} \end{equation} To reduce the second degree equation to a first degree equation we are going to suppose that 0.1>>2s. This approximation is good if the solubility is very low.
\begin{equation} s(0.1+\cancel{2s})^2=7.1x10^{-9}\rightarrow s(0.1)^2=7.1x10^{-9}\rightarrow s=7.1x10^{- 7} \end{equation} In the presence of the common ion the solubility drops to $7.1x10^{-7}$ while in pure water it is $1.2x10^{-3}$



