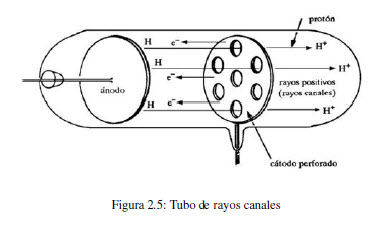
In 1886, Eugen Goldstein (1850-1930) observed that in a cathode ray tube, with a perforated anode, a stream of particles was generated moving from the cathode to the anode. These positive rays come from atoms contained in the tube that have lost electrons. By changing the gas contained in the tube, a change in the e/m ratio of the positive particle is observed. Studies carried out with different gases showed that the charge of ions is a multiple of a value, the unit of positive charge, called a proton.