The electronic configuration of atoms is key to understanding how molecules are formed. Gilbert Newton Lewis concluded that atoms combine to achieve the electronic configuration of the noble gases.
Only the outermost electrons, called valence electrons, participate in the formation of a bond between two atoms. Lewis designed a system to represent the atom with its valence electrons, which consists of drawing the symbol of the element surrounded by dots, which represent each of the valence electrons.
Main ideas of Lewis's theory:
- Valence electrons are responsible for forming bonds.
- Electrons can be transferred between atoms giving rise to cations and anions that attract each other to form ionic compounds.
- When electrons are shared between atoms, covalent bonds are formed.
- The exchanged electrons allow the atoms to acquire the electronic structure of noble gas. They are generally surrounded by 8 electrons in their outer shell, called an octet.
We can easily know the number of valence electrons in an element by looking at the group of the periodic table to which it belongs.
Group 1 (alkalines) have one valence electron.
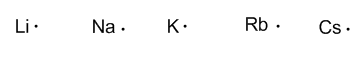
Group 2 (alkaline earth) have two valence electrons.
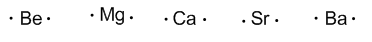
Group 13 (Boron, Aluminum, Gallium, Indium, and Thallium) has three valence electrons.
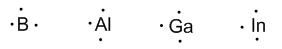
Group 14 (carbon, silicon, germanium, tin, and lead) has four valence electrons.
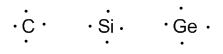
Group 15 (nitrogen, phosphorous, arsenic, antimony, and bismuth) has 5 valence electrons.
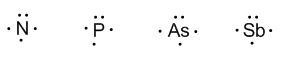
Group 16 (oxygen, sulfur, selenium, and tellurium) has 6 valence electrons.
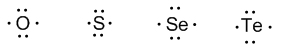
Group 17 (fluorine, chlorine, bromine, and iodine) has seven valence electrons.
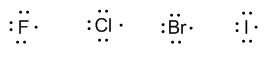
Group 18 (Neon, argon, krypton, xenol, and radon) has eight valence electrons. The only exception to this rule is Helium with two valence electrons.



